Trial drug can significantly block early stages of COVID-19 in engineered human tissues
An international team led by University of British Columbia researcher Dr. Josef Penninger has found a trial drug that effectively blocks the cellular door SARS-CoV-2 uses to infect its hosts.
The findings, published today in Cell, hold promise as a treatment capable of stopping early infection of the novel coronavirus that, as of April 2, has affected more than 981,000 people and claimed the lives of 50,000 people worldwide.
The study provides new insights into key aspects of SARS-CoV-2, the virus that causes COVID-19, and its interactions on a cellular level, as well as how the virus can infect blood vessels and kidneys.
"We are hopeful our results have implications for the development of a novel drug for the treatment of this unprecedented pandemic," says Penninger, professor in UBC's faculty of medicine, director of the Life Sciences Institute and the Canada 150 Research Chair in Functional Genetics at UBC.
"This work stems from an amazing collaboration among academic researchers and companies, including Dr. Ryan Conder's gastrointestinal group at STEMCELL Technologies in Vancouver, Nuria Montserrat in Spain, Drs. Haibo Zhang and Art Slutsky from Toronto and especially Ali Mirazimi's infectious biology team in Sweden, who have been working tirelessly day and night for weeks to better understand the pathology of this disease and to provide breakthrough therapeutic options."
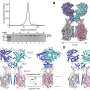
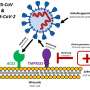
ACE2—a protein on the surface of the cell membrane—is now at centre-stage in this outbreak as the key receptor for the spike glycoprotein of SARS-CoV-2. In earlier work, Penninger and colleagues at the University of Toronto and the Institute of Molecular Biology in Vienna first identified ACE2, and found that in living organisms, ACE2 is the key receptor for SARS, the viral respiratory illness recognized as a global threat in 2003. His laboratory also went on to link the protein to both cardiovascular disease and lung failure.
While the COVID-19 outbreak continues to spread around the globe, the absence of a clinically proven antiviral therapy or a treatment specifically targeting the critical SARS-CoV-2 receptor ACE2 on a molecular level has meant an empty arsenal for health care providers struggling to treat severe cases of COVID-19.
"Our new study provides very much needed direct evidence that a drug—called APN01 (human recombinant soluble angiotensin-converting enzyme 2—hrsACE2)—soon to be tested in clinical trials by the European biotech company Apeiron Biologics, is useful as an antiviral therapy for COVID-19," says Dr. Art Slutsky, a scientist at the Keenan Research Centre for Biomedical Science of St. Michael's Hospital and professor at the University of Toronto who is a collaborator on the study.
In cell cultures analyzed in the current study, hrsACE2 inhibited the coronavirus load by a factor of 1,000-5,000. In engineered replicas of human blood vessel and kidneys—organoids grown from human stem cells—the researchers demonstrated that the virus can directly infect and duplicate itself in these tissues. This provides important information on the development of the disease and the fact that severe cases of COVID-19 present with multi-organ failure and evidence of cardiovascular damage. Clinical grade hrsACE2 also reduced the SARS-CoV-2 infection in these engineered human tissues.
"Using organoids allows us to test in a very agile way treatments that are already being used for other diseases, or that are close to being validated. In these moments in which time is short, human organoids save the time that we would spend to test a new drug in the human setting," says Núria Montserrat, ICREA professor at the Institute for Bioengineering of Catalonia in Spain.
"The virus causing COVID-19 is a close sibling to the first SARS virus," adds Penninger. "Our previous work has helped to rapidly identify ACE2 as the entry gate for SARS-CoV-2, which explains a lot about the disease. Now we know that a soluble form of ACE2 that catches the virus away, could be indeed a very rational therapy that specifically targets the gate the virus must take to infect us. There is hope for this horrible pandemic."
More information: Cell (2020). DOI: 10.1016/j.cell.2020.04.004 , www.cell.com/pb-assets/product … _CELL-D-20-00739.pdf