Experts outline plan for COVID-19 vaccines
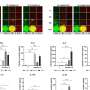
Unprecedented collaboration and resources will be required to research and develop safe and effective vaccines for COVID-19 that can be manufactured and delivered in the scale of billions of doses to people globally.
Vaccine development often takes years. To speed up the process, Dr. Larry Corey of Fred Hutchinson Cancer Research Center, and experts at the National Institutes of Health outline a vision to create a coordinated and efficient approach to creating COVID-19 vaccines.
In a perspective published online May 11 by the journal Science, Corey and coauthors Dr. Anthony Fauci, Dr. John Mascola, and Dr. Francis Collins, share their plan for bringing together industry, government and academia to meet this urgent need. Dr. Fauci is the director of the National Institute of Allergy and Infectious diseases, part of NIH. Dr. Mascola is the director of the NIAID Vaccine Research Center, and Dr. Collins is the NIH director.
"We're experiencing a series of unprecedented events with a disease that has spread globally and infected more people in a shorter time than any other infection in modern times," said Corey, past president and director of Fred Hutch and a professor in its Vaccine and Infectious Disease Division. "In order to overcome the challenges in front of us, we each need to bring nothing short of our absolute best. The research and development of COVID-19 vaccines will require creativity, cooperation and commitment to save as many lives as possible as soon as we can."
Throughout his career in developing vaccines, therapeutics and immunotherapies, Corey has led the successful integration of academic institutions, biotechnology and pharmaceutical companies in collaboration with public funding from NIH.
"Besides scientists and health experts, we need the help of industry and affected communities throughout the country to participate in large-scale trials of potential COVID vaccines," he said.
The authors call for harmonizing each step of the process, from creating a common oversight body and shared set of criteria to evaluate the vaccine studies underway, to transparency and data sharing, to marshalling the full resources of private, public and philanthropic sectors to scale up eventual manufacturing capacity and distribution chains for COVID-19 vaccines.
"We want to see multiple successful vaccines and vaccine platforms meet the global need of immunizing billions of adults, children and restoring economic and health to the world," urged Corey.
More information: Science (2020). science.sciencemag.org/cgi/doi … 1126/science.abc5312