Insulins available at US pharmacies are consistent with product labeling
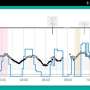
JDRF, the American Diabetes Association (ADA), and The Leona M. and Harry B. Helmsley Charitable Trust, announce the results of a study examining the consistency and potency of insulin purchased at U.S. retail pharmacies. The study, led by University of Florida researcher Timothy Garrett and published in Diabetes Care, found all human and analog insulins measured contained the expected quantity of active insulin.
Insulin is a life-saving drug for people with diabetes that can be dangerous and potentially fatal when incorrectly dosed. Variation in insulin activity or insufficient insulin activity would pose significant challenges and safety risks for people with diabetes attempting to manage their blood glucose levels.
"During such an unprecedented time it's more important than ever for people with T1D to feel safe. This study reaffirms our confidence in the safety and effectiveness of insulin products," said Aaron Kowalski, Ph.D., president and CEO of JDRF. "As JDRF, our partners, and the type 1 diabetes community pursue cures and support the development of better insulins, we must also work together to make these safe and effective insulin products affordable and accessible to everyone who needs them."
"People living with type 1 diabetes make a life or death decision each time they decide how much insulin to dose, which happens many times a day. Having confidence in insulin quality is paramount," said David Panzirer, a Trustee at the Helmsley Charitable Trust, and a parent of two children with type 1 diabetes. "Helmsley is committed to easing the burden of living with type 1 diabetes, and this study brings welcomed good news to our community and should alleviate any lingering concern around the quality of our insulin supply."
In April 2018, JDRF, ADA, and Helmsley issued a request for proposals (RFP) to study insulin potency and consistency, which was prompted by a small study published in 2017 that found variation in the level of active insulin in products available commercially in the United States. Notably, this 2017 study did not use a research method approved by the U.S. Pharmacopeia (USP) and was inconsistent with data from previous regulatory audits. To research the issue further, JDRF, ADA, and Helmsley supported a team that devised an unbiased, well-powered, and independent assessment of insulin products from major manufacturers using approved USP methods.
USP and the U.S. Food and Drug Administration require insulin vials and cartridges to contain 100 U/mL (± 5 U/mL).
This single year of research will be expanded during a second study phase to measure any potential seasonal variations in reported insulin activity.
More information: Timothy J. Garrett et al, Commercially Available Insulin Products Demonstrate Stability Throughout the Cold Supply Chain Across the U.S., Diabetes Care (2020). DOI: 10.2337/dc19-1941