Trial validates a less intense treatment for HPV+ throat cancer
The final results of a randomized phase two trial E3311 will be presented at the American Society of Clinical Oncology (ASCO) annual meeting, to occur virtually from May 29-31. The trial, conducted in patients undergoing transoral robotic surgery (TORS), tested reduced postoperative radiation therapy in patients with human papillomavirus-associated oropharynx squamous cell carcinoma at intermediate risk for recurrence. ASCO is highlighting the importance of the positive results by making it the first presentation in its Head and Neck Oral Abstract Session (Abstract 6500). The ECOG-ACRIN Cancer Research Group designed and conducted the trial with funding from the National Cancer Institute, part of the National Institutes of Health.
"Transoral resection followed by low-dose radiation is safe in patients with intermediate-risk locally advanced oropharynx cancer, with very good oncologic outcome," said lead investigator Robert L. Ferris, MD, Ph.D., director, UPMC Hillman Cancer Center in Pittsburgh and a surgical oncologist specializing in head and neck cancer (pictured). "These results present a promising deintensification approach."
Most pharynx cancers caused by HPV have a very good outcome, and the cancer does not return or spread to other parts of the body after treatment. Dr. Ferris and colleagues sought to prove the benefits of using tumor pathologic features, obtained in specimens collected at surgery, to determine patients' risk of recurrence-low, intermediate or high. In particular, they sought to more clearly define the prognostic and predictive role of traditional pathologic biomarkers such as extensive nodal or extranodal disease, to give the right amount of postoperative treatment for each risk group.
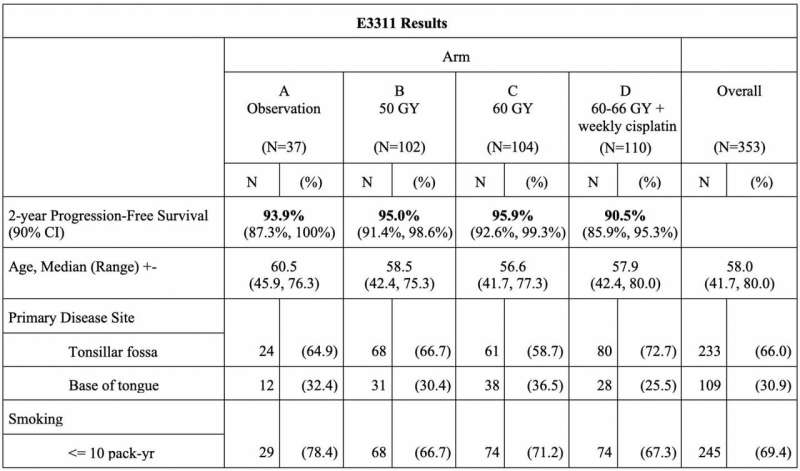
Patients at low risk were observed. Patients at intermediate risk were randomized to two arms of radiation alone, both at doses lower (50Gy or 60Gy) than usual (60-66Gy). At the time the trial opened in 2013, the optimal dose of radiation therapy was not defined. Patients at high risk were assigned to usual radiation therapy plus chemotherapy.
"Study E3311 met its primary endpoint," said Dr. Ferris. "For intermediate risk patients-those with uninvolved surgical margins, less than five involved nodes, and less than 1mm extranodal extension-reduced-dose postoperative radiation therapy without chemotherapy appears sufficient. In our study, this group had better outcomes than the group on usual high-dose radiation plus chemotherapy, showing that our patient stratification identified low and intermediate risk patients well, preserving patients' throat function and sparing them unnecessary short- and long-term toxicities."
For patients with low-risk disease, two-year progression-free survival (PFS) was favorable without postoperative therapy (observation alone). All arms had two-year survival rates above 90% and there was no excess of local recurrences with reduction in radiation or chemotherapy (Arms B and C). Risk stratification appeared to appropriately select patients for observation (Arm A).
The overall intent of E3311 was to gather essential data for the design of a future, randomized phase three trial. The primary endpoint was to determine the feasibility and oncologic efficacy of a prospective multi-institutional study of TORS for HPV+ oropharynx cancer followed by risk-adjusted adjuvant therapy. The primary endpoint was two-year progression-free survival in patients determined to be at intermediate risk after surgical excision.
"The tissue samples and imaging studies collected in the course of this trial are a rich resource for studying the biology of intermediate- and high-risk disease, in work that is ongoing," said ECOG-ACRIN Head and Neck Committee Chair, Barbara A. Burtness, MD, Professor of Medicine, and Co-Leader, Developmental Therapeutics Program, Yale Cancer Center and Yale School of Medicine (pictured). "ECOG-ACRIN plans to pursue the current data with a randomized phase three trial of TORS-based treatment deintensification compared with conventional chemoradiation."
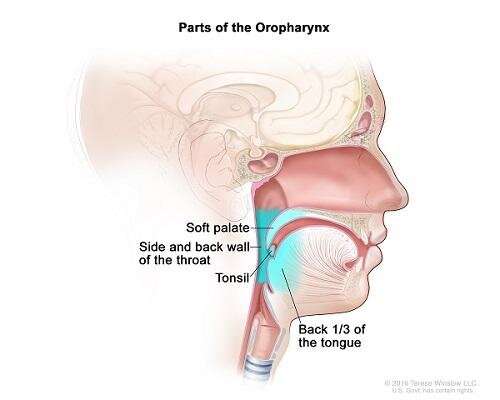
A lump in the neck and a sore throat are the most common signs of oropharynx cancer, a disease in which cancer cells form in the tissues of the oropharynx-the middle part of the throat (pharynx), at the base of the tongue and tonsils. About 60% of oropharynx cancers are associated with HPV infection. The incidence has been increasing in recent years, especially in individuals under the age of 45. This change is attributed to the increasing prevalence of HPV infection in developed countries, the practice of oral sex, and the rising number of sexual partners.
While surgeons and patients widely favor the organ-preservation approach of transoral robotic surgery, there remain serious concerns about both short- and long-term toxicities associated with chemotherapy. Besides, given the potential long-term consequences of radiation therapy for a younger population, re-evaluation of adjuvant treatment intensity is needed.