This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Genetically modified mice reveal protein receptor's role in metabolic health
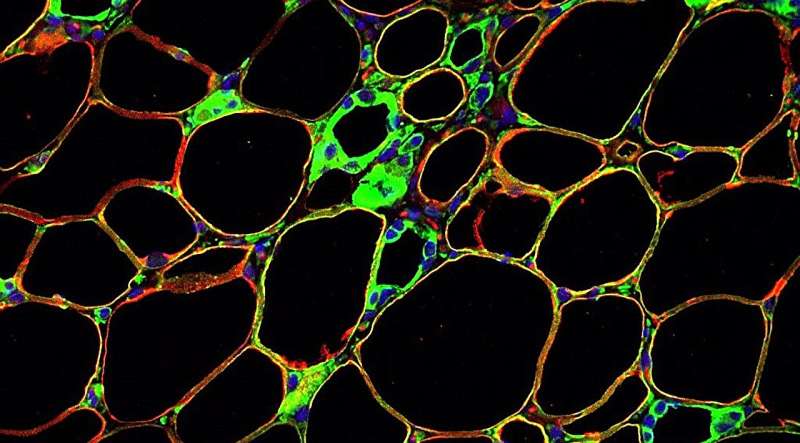
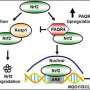
A protein receptor called PAQR4 found within fat cells appears to act as a sensor for ceramides, waxy lipids whose overabundance has been linked to a variety of metabolic disorders and cancers, a study led by UT Southwestern Medical Center researchers suggests.
Their findings, published in Nature Metabolism, could eventually lead to drugs that reduce cellular ceramide levels, much like statins reduce cholesterol levels.
"Rising ceramides in the cell are responsible for inflammation, insulin resistance, and cell death," said study leader Philipp Scherer, Ph.D., Professor of Internal Medicine and Cell Biology and Director of the Touchstone Center for Diabetes Research at UT Southwestern.
"Just like cholesterol, excessive levels of ceramides are particularly bad for the cell and metabolism at large. Defining a cellular mechanism by which levels of ceramides are sensed is therefore quite important."
Ceramides play critical roles in tissues throughout the body. They help the skin form a moisture-retaining barrier and insulate nerves, for example. However, their overabundance is tied to several conditions, including type 2 diabetes, cardiovascular disease, and multiple malignancy types including skin, non-small cell lung, prostate, and breast cancers.
For the last three decades, the Scherer Lab has studied molecular pathways related to adiponectin, a hormone with roles in various metabolic processes. The hormone is also known for its healthful insulin-sensitizing and anti-inflammatory effects. Adiponectin exerts these effects by binding to two receptors known as PAQR1 and PAQR2 present on the surface of fat cells. PAQR4, a receptor in the same protein family, has an extremely similar structure; however, its function has been unknown.
Wondering whether PAQR4 might also interact with adiponectin to provide health benefits, the researchers investigated its role by genetically modifying lab mice so that they overproduced PAQR4. Despite no change in their food intake, these animals lost weight when carrying higher levels of PAQR4.
While weight loss is often an indicator of improved metabolic health, these animals were far from healthy. Their blood sugar and insulin production sharply increased, suggesting that they had developed diabetes, and their livers became loaded with excess fat.
When Qingzhang Zhu, Ph.D., first author on the study and Instructor of Internal Medicine at UTSW, genetically modified other lab mice to shut off PAQR4 production, their metabolic health improved. Similar effects occurred when using a drug to block cellular production of ceramides.
Additional experiments showed that PAQR4 appears to detect the quantity of ceramides in fat cells. In turn, by mediating the stability of proteins that synthesize ceramides, PAQR4 regulates how much of these lipids are present in cells, hence acting as an important sensor for the levels of cellular ceramides.
Dr. Scherer said further research will look at ways to target PAQR4 with drugs that decrease its function and consequently stymie ceramide production, which could treat or prevent these conditions.
More information: Qingzhang Zhu et al, PAQR4 regulates adipocyte function and systemic metabolic health by mediating ceramide levels, Nature Metabolism (2024). DOI: 10.1038/s42255-024-01078-9