Identifying antibodies that work
A new test manufactured by A*STAR's Diagnostics Development Hub in partnership with Duke-NUS Medical School could reveal if an individual is likely immune to COVID-19.
After months of quiet, countries from Singapore to Spain are emerging from unprecedented lockdowns brought on by COVID-19. Public spaces are buzzing with activity and filled with people once more, providing a much-needed boost to morale—and the economy.
Though it may seem like things are finally going back to the status quo, the pandemic is far from over. Instead of being complacent, we should be more vigilant than ever. To safely guide the influx of people back to the workplace and other venues, some governments have floated the idea of providing "immunity passports" to those who have recovered from the coronavirus.
Given that approximately 40 to 45% of COVID-19 cases are asymptomatic, the true scale of the pandemic has so far remained elusive. Consequently, widespread antibody testing has been suggested as a means to identify individuals who could be given these immunity passports, resulting in the market being flooded with rapid antibody tests. Unfortunately, not only are some of these tests wildly inaccurate, they cannot indicate immunity to reinfection.
Enter the cPass kit, a first-of-its-kind rapid test that specifically detects the functional neutralizing antibodies that can clear coronavirus infection. Unlike RT-PCR tests, cPass cannot be used to diagnose an ongoing infection. Instead, it indicates if an individual had previously been infected and developed protective antibodies in the process. Invented by Professor Wang Linfa and his team at the Duke-NUS Medical School (Duke-NUS), the kit's commercialization was co-facilitated by the GenScript Biotech Corporation and Diagnostics Development (DxD) Hub at Singapore's Agency for Science, Technology and Research (A*STAR).
"Unlike RT-PCR tests, the main use of cPass is not to diagnose an ongoing infection. Instead, it indicates if an individual had previously been infected and developed protective antibodies in the process. This will be a huge boost to current COVID-19 investigations, to determine infection rate, herd immunity, predicted humoral protection, and also vaccine efficacy during clinical trials and after large-scale vaccination," said Wang, director of the Duke-NUS' Emerging Infectious Diseases program.
Antibodies 101
When a person is infected with SARS-CoV-2, the virus that causes COVID-19, the body mounts an immune response, triggering the production of hundreds to thousands of different antibodies in the blood. While SARS-CoV-2-specific antibodies can recognize and bind to the virus, not all of them can prevent it from invading cells. Some antibodies, for instance, could recognize parts of the coronavirus, but fail to functionally reduce its infection.
These so-called non-neutralizing antibodies may even paradoxically enhance subsequent viral reinfection in a phenomenon known as antibody-dependent enhancement. To guarantee protection against future SARS-CoV-2 infection, we need to look for neutralizing antibodies that mainly bind to the coronavirus' spike protein. By doing so, these antibodies block the spike protein from attaching to the angiotensin-converting enzyme 2 (ACE2) receptor found on the surface of host cells, thereby preventing the virus from infiltrating the cell and replicating within.
Though rapid tests can detect the presence of antibodies against SARS-CoV-2, they are unable to distinguish between the neutralizing and non-neutralizing antibodies. Testing specifically for neutralizing antibodies has proven to be tricky. After all, these tests are based on the blocking of the live virus by neutralizing antibodies present in the cell culture setups. Not only does the process require skilled laboratory personnel and high-level biocontainment facilities, but it also takes several days to complete—making widespread and large scale neutralizing antibody testing a pipe dream. At least, until now.
Neutralizing or not?
With the cPass kit, neutralizing antibodies can be detected within an hour in most research and clinical laboratories. In contrast to the other tests, cPass does not expose laboratory personnel to potentially dangerous live biological materials. Moreover, the kit can be easily scaled up and fully automated. "It fills a space in the market where no solution exists," remarked Dr. Sidney Yee, CEO of A*STAR's DxD Hub.
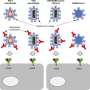
For SARS-CoV-2 to infect cells, it needs to bind to a protein called ACE2. It does this using the spike proteins that give coronaviruses their name, specifically a part of the spike protein called the receptor-binding domain (RBD). If a person has neutralizing antibodies against SARS-CoV-2, they will prevent the RBD from binding to ACE2, effectively preventing the virus from entering the cell.
The cPass kit mimics this virus neutralization process in the lab. The kit contains two key components, namely the RBD protein fragment that have been labeled with an enzyme and the ACE2 protein coated on a plate. In the absence of neutralizing antibodies, the RBD fragments will bind to ACE2, producing a color after exposing to an enzyme substrate.
If neutralizing antibodies are present, however, the RBD fragments are prevented from binding to the ACE2 on the plate and no or less color is formed. By measuring the intensity of color produced by each reaction and comparing it to controls, users can thus determine if their sample contains neutralizing antibodies or not. So far, the cPass kit has achieved sensitivity and specificity rates of close to 100%—with its performance far outstripping other antibody tests on the market.
One kit, many uses
Just like the Fortitude RT-PCR diagnostic kit, the development of cPass was an all-out collaborative effort, this time involving the Duke-NUS Medical School, A*STAR's DxD Hub and GenScript. According to Yee, the whole process from conception, patent filing, kit development to provisional authorization from the local Health Sciences Authority (HSA) took less than three months, with GenScript assisting in proof-of-concept research, product design and optimization.
To help secure provisional authorization for the kit and to bring it to the market, the DxD Hub validated the kit using local COVID-19 patient samples from the PROTECT clinical study coordinated by Singapore's National Center for Infectious Diseases. In addition, the DxD team also developed the manufacturing and quality control protocols, producing the first pilot batch for use in local hospitals. GenScript and local Singapore biotech companies are set to scale up production of the kit.
Aside from Singapore, groups from many countries including Malaysia, Thailand, Vietnam, Sri Lanka, China, Australia, New Zealand, U.S., Canada, Germany, UK, the Netherlands and Switzerland are already testing the kit. Approval applications are also being filed in Europe and the US, with GenScript even building up their production lines in China and the US in anticipation of the demand—and with good reason. Beyond COVID-19 surveillance, the kit can be used broadly for everything from assessing blood samples for convalescent plasma therapy to tracking coronavirus infections in animals, said Prof Wang.
Besides, cPass could be used to calculate just how long the neutralizing antibodies confer protective immunity—a question that has been continuously debated in scientific circles since the beginning of the outbreak. As the race for a vaccine kicks into high gear, pharmaceutical companies will also find the kit useful for determining the efficacy of their candidate with a few vaccine companies already in the process of evaluating cPass for this purpose.