It's time to include children in COVID-19 vaccine trials
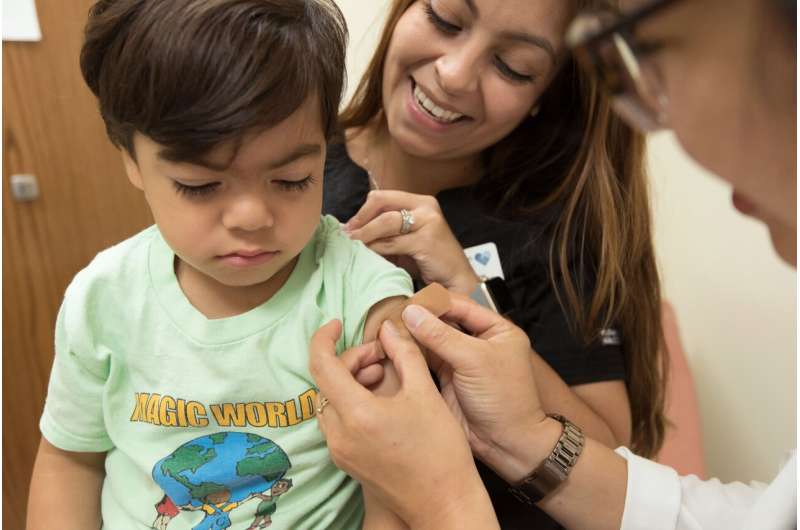
When it comes to COVID-19 vaccine development, don't leave kids behind. This is the message from Sharon Nachman, MD, chief of the Division of Pediatric Infectious Diseases at Stony Brook Children's Hospital, and her co-authors on the newly published paper in Clinical Infectious Diseases titled: Warp Speed for COVID-19 Vaccines: Why are Children Stuck in Neutral?
"We need to start evaluating the different vaccines now and in the context of pediatric use, because if vaccines are approved and we don't know how well they work for children that puts children at a higher risk for infection and further disease spread," said Dr. Nachman.
She emphasizes that the "now" is essential in these times, as generally it takes up to eight years to license a vaccine used in adults then accommodated to license the vaccine in children.
"If we follow the usual model, we will be in trouble and never be able to modify the COVID-19 pandemic because we will have a huge swath of the population about whom we know nothing in regards to the infection and vaccination."
The team of national pediatric infectious disease experts authoring the paper articulate: "While adult clinical trials of COVID-19 vaccines have moved quickly into Phase 3 clinical trials, clinical trials have not started in children in the US. The direct COVID-19 impact upon children is greater than that observed for a number of other pathogens for which we now have effective pediatric vaccines. Additionally, the role of children in SARS-CoV-2 transmission has clearly been underappreciated. Carefully conducted Phase II clinical trials can adequately address potential COVID-19 vaccine safety concerns. Delaying Phase II vaccine clinical trials in children will delay our recovery from COVID-19 and unnecessarily prolong its impact upon children's education, health and emotional well-being, and equitable access to opportunities for development and social success. Given the potential direct and indirect benefits of pediatric vaccination, implementation of Phase II clinical trials for COVID-19 vaccines should begin now."
More information: Evan J Anderson et al. Warp Speed for COVID-19 Vaccines: Why are Children Stuck in Neutral?, Clinical Infectious Diseases (2020). DOI: 10.1093/cid/ciaa1425