Novel mechanical mechanism of metastatic cancer cells in substrates of different stiffness revealed
During metastasis, cancer cells actively interact with microenvironments of new tissues. How metastatic cancer cells respond to new environments in the secondary tissues is a crucial question in cancer research but still remains elusive. Recently, researchers from the Hong Kong University of Science and Technology (HKUST), along with their international collaborators, discovered a novel mechanical mechanism of metastatic cancer cells in substrates of different stiffness, which could contribute to developing diagnostic tools for metastatic cancer cells and cancer therapeutics.
This study was published in the Journal of Physical Chemistry Letters on Sept 18, 2020.
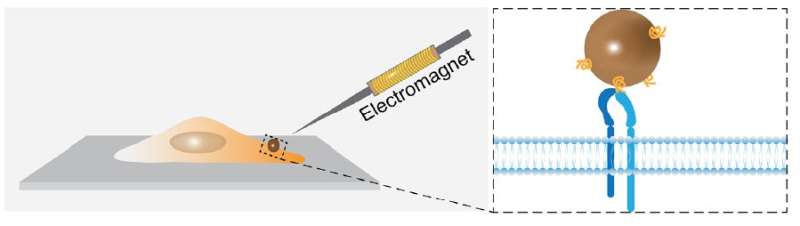
In the study, the team of researchers, led by Prof. Hyokeun Park, assistant professor at the Department of Physics and Division of Life Science, HKUST, mimicked mechanical stiffness of diverse tissues from soft brain to bone using polyacrylamide (PAA) substrates and measured the mechanical responses of single metastatic breast cancer cells (MDA-MB-231 cells) against different stiffness, using advanced imaging techniques and the state-of-the-art magnetic tweezers which Prof. Park's group built in HKUST.
Using single-molecule tension sensors, they found that metastatic breast cancer cells change their tension in focal adhesions against the stiffness to adapt new environments whereas normal breast cells (MCF-10A cells) keep the similar tension regardless of stiffness. They also measured the viscoelasticity of single metastatic breast cancer cells using magnetic tweezers and found that metastatic cancer cells become more elastic on stiffer substrates while the viscoelasticity of normal cells remain similar.
These results show that metastatic breast cancer cells have stronger capacity to adapt to the mechanical environments of diverse tissues.
"How do metastatic breast cancer cells migrate and proliferate the secondary tissues of different stiffness from soft to hard tissue interface like brain to bone is a crucial question in cancer research," said Prof. Park. "Our work addressed how metastatic breast cancer cells proliferate the substrate of varying stiffness from 1kPa (similar to brain) to 50GPa (similar to bone), and we discovered that metastatic cancer cells change their viscoelasticity depending on physical environment to adapt their new physical environment and survive their new environment. This is one of big achievements in cancer physics and mechanobiology. The findings will contribute to developing diagnostic tools for metastatic cancer cells and, eventually, treatment of cancer."
This work was done in collaboration with Prof. Ching-Hwa Kiang at Rice University, Professor Jun Chu at Shenzhen Institutes of Advanced Technology and Prof. Ophelia Tsui in Department of Physics of HKUST.
The team is planning to develop a cancer diagnostic kit making use of the mechanism to measure tension of living potential cancer cells at different stiffness, which will be much simpler and more user-friendly than the existing diagnosis tools for metastatic cancer cells. Such mechanism could also be used to develop a drug-screening test for metastatic cancer to see how metastatic cancer cells' focal adhesion and viscoelasticity respond to different drugs and find the most effective drug for them.
More information: Fang Tian et al, Mechanical Responses of Breast Cancer Cells to Substrates of Varying Stiffness Revealed by Single-Cell Measurements, The Journal of Physical Chemistry Letters (2020). DOI: 10.1021/acs.jpclett.0c02065