Highly efficient photodynamic-immunotherapy by combining AIEgen with Poly(I:C)
Immunotherapy is a type of anti-tumor treatment and has shown great clinical success against a wide variety of malignancies in recent years. Poly(I : C), a TLR3 agonist, is the most potent type I interferon (IFN) inducer. Poly(I : C) not only directly induces tumorous apoptosis, but also stimulates tumor cells to secrete immune factors. However, the immune response rate induced by Poly(I : C) remains low in several types of malignancies and higher doses are often required to achieve the desired effect. However, poly(I : C) is highly toxic and thus only a very narrow therapeutic window is available, which greatly limits clinical application of Poly(I : C)-based treatments.
Photodynamics therapy (PDT) is a promising anti-tumor treatment method, which not only directly induces the production of excessive reactive oxygen species (ROS) in tumor cells, but also promotes the release of tumor-related antigens, thereby initiating the anti-tumor immunity. The photosensitive activity of traditional photosensitizers is weakened or even completely disappeared in the state of aggregation, which is a major problem in PDT. Fortunately, the emergence of AIEgens presents a new strategy for the construction of effective photosensitizers. The AIEgens have a stronger ability to generate ROS and produces a stronger immune effect in the aggregated state. Even so, the immunity induced by photodynamic therapy is still difficult to achieve complete tumor elimination. Therefore, combining the immune adjuvant Poly(I:C) with AIE photosensitizer-mediated photodynamic therapy to enhance the anti-tumor immune response is of great clinical significance.
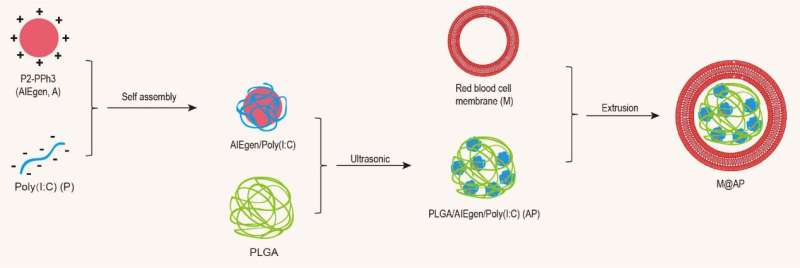
Recently, Shixuan Wang from Huazhong University of Science and Technology of China, Fan Xia from the China University of Geosciences and Yuning Hong the La Trobe University of Australia reported the preparation of NPs that comprise an AIE-conjugated polymer as a photosensitizer and Poly(I : C) as an immunologic adjuvant, a PLGA matrix and an red blood cell (RBC) membrane shell, which provide a novel strategy for tumor treatment.
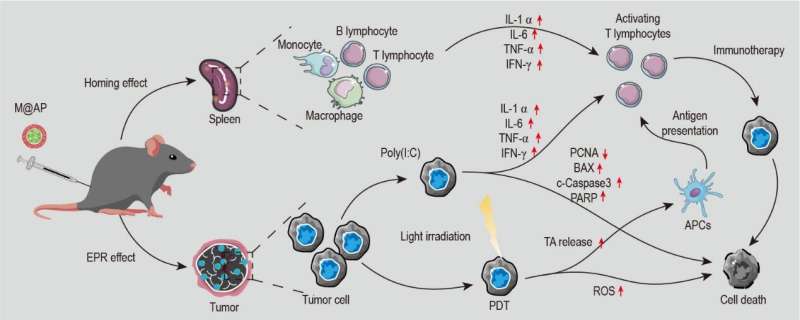
In order to enhance the good biocompatibility and specific organ targeting of the NPs, the authors used the red blood cell membrane as the shell, simultaneously loaded the AIEgens and the immune adjuvant Poly(I:C) to construct the M@AP NPs. In vivo, M@AP NPs are mainly enriched in tumor tissues because of the ERP effect, but they also accumulate in the spleen through the homing effect of the RBC membrane. The latter activates immune cells in the spleen to strengthen anti-tumor immunity. Under white light irradiation on the tumor region, the AIE photosensitizer induces tumor cell death by generating intracellular ROS, which further promotes the release of TA and activates the immune response with a synergistic effect of Poly(I : C). Quantitative analysis revealed that M@AP NPs simultaneously kill local cancer cells directly and stimulate the immune cells to release cytokines that present cytotoxicity against tumor cells. The authors further applied the system in a lung metastasis mouse model and demonstrated the excellent anti-metastasis capability of our approach, which provides a potential treatment strategy for preventing tumor recurrence and metastasis.
More information: Jun Dai et al, Red blood cell membrane-camouflaged nanoparticles loaded with AIEgen and Poly(I: C) for enhanced tumoral photodynamic-immunotherapy, National Science Review (2021). DOI: 10.1093/nsr/nwab039