Early use of biologics recommended in patients with moderate-to-severe Crohn's disease
Crohn's disease, a type of inflammatory bowel disease (IBD) that causes inflammation (pain and swelling) in the gastrointestinal tract, can cause daily health problems, frequent hospitalizations and surgery when not adequately controlled. While there is no cure for Crohn's disease, there are treatments that can help patients live a symptom-free life.
After a detailed review of available literature, the American Gastroenterological Association (AGA) has released new clinical guidelines outlining the benefits and risks of each drug currently available to Crohn's patients. Based on this research, AGA recommends the early introduction of biologics for patients experiencing luminal and fistulizing Crohn's disease rather than waiting until other treatments fail. These guidelines are published in Gastroenterology, AGA's official journal.
"With many new drugs entering the market, clinician's ability to treat patients with Crohn's disease has improved greatly over the last 20 years," said lead author Joseph D. Feuerstein, MD, from Beth Israel Deaconess, Boston, Massachusetts. "We hope this new guideline serves as a manual for clinicians in selecting the right therapies for their patients, which should lead to improved patient outcomes and less need for invasive surgery."
Key guideline recommendations:
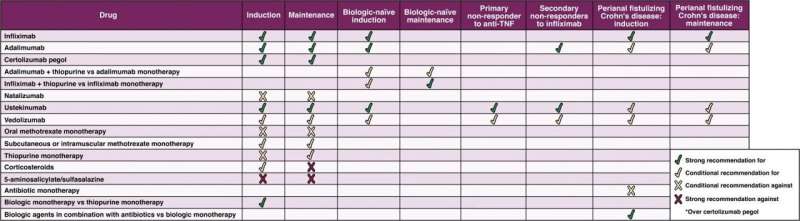
Biologics are the most effective drugs for the management of Crohn's and they should be used early, rather than delaying their use until after failure of mesalamine and/or corticosteroids, in patients with moderate to severe or fistulizing Crohn's disease. These drugs are antibodies and can more precisely target the immune system which is causing the inflammation in Crohn's disease.
a. Anti-tumor necrosis factor (anti-TNF) agents or ustekinumab are recommended and vedolizumab is suggested as a first-line treatment.
b. In patients who have previously not responded to anti-TNF agents, AGA recommends ustekinumab or vedolizumab.
c. The biologic natalizumab is no longer recommended due to potential adverse events and the availability of safer treatment options.
Read the AGA Clinical Practice Guideline on the Medical Management of Moderate to Severe Luminal and Fistulizing Crohn's Disease to review all 25 recommendations.
More information: Joseph D. Feuerstein et al, AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn's Disease, Gastroenterology (2021). DOI: 10.1053/j.gastro.2021.04.022