July 26, 2021 feature
Is it possible to deliver a knockout punch to HIV?
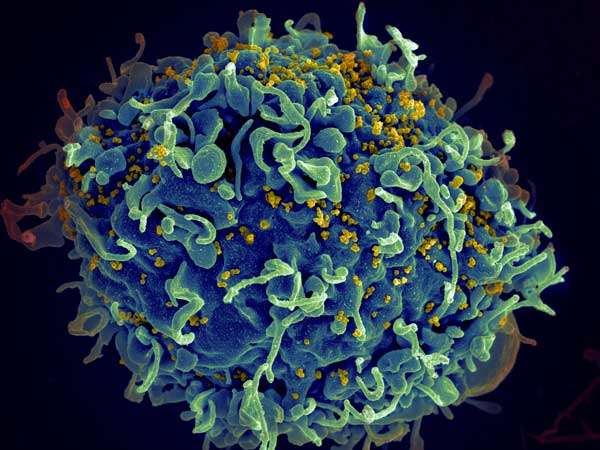
With a global focus on strategies to curb expansion of a fast-moving coronavirus pandemic, the question again has arisen: What more is being done about HIV, a scourge that has lasted more than 40 years—is a cure finally in sight?
Although many antiretroviral medications have been approved over the years, new strategies are under development that theoretically could deliver a knockout blow to HIV. Medical investigators are exploring the possibility of gene therapy as a potential HIV cure. Other teams are examining CAR T-cell therapy, a form of immunotherapy that has demonstrated effectiveness against certain forms of cancer. Technically known as chimeric antigen receptor-modified T cell therapy, the method involves extracting T cells from a patient's blood, followed by modifying them in a laboratory to recognize and destroy HIV-infected cells.
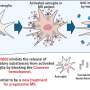
But there are other efforts still, and a new proof-of-concept trial might pave the way for further studies of an experimental immune-boosting compound, which has been tested in combination with coventional antiretroviral therapy, commonly known as ART. The experimental compound—vesatolimod—activates components of the innate and acquired immune systems to put additional pressure on HIV. Vesatolimod essentially marshals a diverse army of immune system fighters in its assault on the human immunodeficiency virus.
Currently, ART can impressively reduce viral loads to undetectable levels in the blood, a feat that some medical investigators call a functional cure. Antiretroviral therapy works by controlling the replication of HIV, and when replication is controlled, the viral load in the blood dramatically diminishes. But HIV can hide in the body, persisting in a stubborn latent state that's impervious to ART. The virus can also rebound prolifically when patients stop taking ART.
Writing in Science Translational Medicine, medical investigators from Gilead Sciences, a pharmaceutical company in Foster City, Calif., and collaborators from leading research centers throughout the United States, describe their small Phase 1b clinical study of vesatolimod. The experimental compound works by ramping up key members of the innate immune system: Interferon, proteins that "interfere" with viruses to block their replication; and natural killer cells, immune constituents whose name aptly describes their function. The compound also has a hand in T cell activation.
"Compared with placebo, vesatolimod was associated with an increase in interferon signaling and natural killer cell and T cell activation, and a decay in the frequency of cells harboring intact HIV genomes," wrote Dr. Devi SenGupta of Gilead Sciences, lead author of the research. "Vesatolimod also induced a modest increase in the time to virus rebound after antiretroviral therapy was interrupted."
Vesatolimod is described as a toll-like receptor 7 agonist, or more simply, a TLR7 agonist. Any agonist initiates a response from a protein receptor. In this case, vesatolimod initiates activity from toll-like receptor 7. Structural biologists have identified more than a dozen distinct toll-like receptors, which are most commonly found stippling the surfaces of macrophages and dendritic cells. When a virus or other pathogenic invader infiltrates cells, toll-like receptors activate innate immune system responses. With an agonist, such as vesatolimod, scientists are targeting a specific toll-like receptor to do a specific job: Assault HIV.
Toll-like receptor agonists are considered a promising class of compounds because in addition to boosting interferon signaling and natural killer cell activity, these compounds as a group are potent dendritic cell activators. Dendritic cells are critical links between the innate and acquired immune systems. T cells, key members of the acquired immune system, have a ramped presence under the influence of the experimental compound.
It's important to underscore: HIV infection is indelibly characterized by the deadly depletion of CD4+ T cells, which are targeted by the virus. While the experimental therapy is in no way capable of capable of boosting CD4+ T cells, it is being investigated for its potential to help eliminate the viral reservoir of HIV by amplifying the immune response.
More than 70 million people have been infected with HIV since the human immunodeficiency pandemic began in the early 1980s, and an estimated 35 million people have died of the infection over the past 40 years, according to UNAIDS, a joint United Nations effort involving 11 UN organizations aimed at combating HIV/AIDS.
UNAIDS estimates that more than 37 million people worldwide are living with HIV, and that ART has helped significantly reduce morbidity and mortality caused by the virus, transforming the infection into a chronic disease for people in many parts of the world.
Vesatolimod, meanwhile, isn't the first toll-like receptor agonist to be used in a clinical study, others have been developed and are being explored in cancer treatment as potent adjuvants—boosters—in cancer vaccine therapy.
In the HIV research, SenGupta and colleagues focused on a unique group of patients known as "controllers." These patients can stop taking their prescribed antiretroviral therapy but, remarkably, even when not on the life-saving medication, they are able to keep HIV replication somewhat under control.
Beyond the investigators' focus on these unusual patients, the research team also wanted to determine how well vesatolimod augments treatment with ART. Hitting the virus with ART and vesatolimod is one way to deliver a one-two punch against HIV. Whether it's a way to deliver a knock-out punch can be answered only through many, many more rounds of research, experts say.
Scientists initially developed vesatolimod to treat chronic hepatitis infections, but it was research beyond those initial studies that produced a eureka moment involving the experimental compound. In animal studies vesatolimod substantially shrank the reservoir of an HIV-like virus—simian immunodeficiency virus—that infects monkeys. After that discovery, scientists began analyses to determine the compound's efficacy as a treatment for HIV.
SenGupta and colleagues recruited 25 HIV "controllers" on ART, gave 17 of them vesatolimod—TLR7—on a biweekly basis, and then halted ART. The remaining 8 received a placebo. Controllers treated with vesatolimod fared better than controllers treated who were given a placebo—but only modestly. Those treated with vesatolimod showed an increase in active immune cells and a significant drop in HIV DNA. Their HIV rebounded a full week after the HIV rebound of the placebo group.
Stunningly, one participant didn't experience an HIV rebound for 15 weeks after taking the oral experimental compound. The team is now calling for larger clinical trials to further evaluate vesatolimod.
SenGupta describes the current research as a proof-of-concept study and suggests that adding vesatolimod to antiretroviral therapy regimens could enhance therapeutic measures to control HIV infection. The experimental compound seems to increase signaling activity and interaction between key components of the innate immune system, according to the research.
"Inferred pathway analysis suggested increased dendritic cell and natural killer cell cross-talk and an increase in cytotoxicity potential after vesatolimod dosing," SenGupta wrote. "Larger clinical studies will be necessary to assess the efficacy of vesatolimod-based combination therapies aimed at long-term control of HIV infection."
For millions of HIV patients worldwide, antiretroviral therapy means taking medication daily—for life. More than 40 drugs have been approved over the years, and some, such the integrase inhibitors, Biktarvy and Duvato, are single-tablet medications. ART not only keeps HIV infections from developing into full-blown AIDS, but viral levels can be driven so low, they are undetectable by laboratory tests. Once viral levels are undetectable, HIV can no longer be sexually transmitted.
Yet, as impressive as that may seem, undetectable does not constitute a cure and some HIV patients—controllers in particular—wax and wane between periods of taking and not taking prescribed ART.
Also, antiretroviral therapy is not without its drawbacks. The medications can cause side effects that range from mild to formidable. For example, skin rashes and weight gain are possible side effects with some HIV medications. High cholesterol levels and heart and kidney problems are among the more serious side effects associated with others.
Still, antiretrovial therapy is considered one of modern medicine's greatest triumphs because it grants HIV patients near-normal life expectancy. Researchers have therefore prioritized finding a cure for HIV that can either eliminate the virus or prevent it from rebounding after patients interrupt their daily regimen of ART. Thus, the pursuit of a compound such as vesatolimod—TLR7—and its ability to prompt an immune response against HIV. Future studies, the team hopes, may produce stronger results.
For now, the proof-of-concept research suggests scientists are moving in the right direction: "The TLR7 agonist vesatolimod induced a modest delay in viral rebound in HIV controllers after cessation of antiretroviral therapy," SenGupta wrote.
More information: Devi SenGupta et al, The TLR7 agonist vesatolimod induced a modest delay in viral rebound in HIV controllers after cessation of antiretroviral therapy, Science Translational Medicine (2021). DOI: 10.1126/scitranslmed.abg3071
© 2021 Science X Network