What mutant flies can teach us about autoimmune disorders
From founding the field of genetics research to unraveling the mysteries of disease, tiny fruit flies have made a big impact on our understanding of human biology.
While it may not look it, the insects share 60% of their genes with humans. Fruit flies have therefore become an important model organism for studying gene function and interactions—helping scientists explore everything from aging to cancer.
According to a study published this week in Proceedings of the National Academy of Sciences, those tiny but mighty flies may also hold an important piece of the autoimmune disease puzzle.
"We focused on a fly mutant that mounts an immune response against one of its own internal organs," said study co-author Todd Schlenke, an associate professor in the Department of Entomology in the University of Arizona College of Agriculture and Life Sciences.
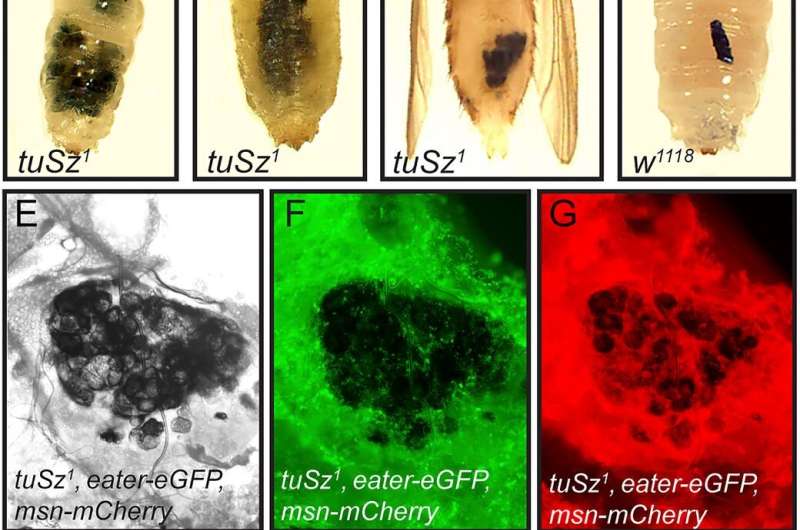
These particular mutant fruit flies, known to the scientific community as tumor Suzuki flies, were first discovered in the 1960s and are recognizable by the gobs of blood cells swarming over what's known as their fat body, which is equivalent to the human liver, Schlenke explained.
Over the course of the last century, fruit flies have become a sort of genetic road map for scientists, with new strains of the insect created with targeted genes turned off and on. Sometimes a new strain is created but the specific underlying gene mutations are unknown. Although the tumor Suzuki flies have been around for several decades, no one has ever linked the mutation to a specific gene.
"It was just such a fascinating class of mutants to me. This whole idea of self-tolerance and autoimmunity is something that I've always been really interested in trying to understand," said Nathan Mortimer, lead study author and an assistant professor of cellular immunology in the School of Biological Sciences at Illinois State University. "Particularly using a simple organism like the fruit fly to look at basic immune functions and understand what role they play in autoimmunity."
Blood cells have multiple, overlapping ways of distinguishing foreign pathogens from host tissues. Recognition of pathogen-specific molecules results in the activation of an immune response against the foreign tissue, while recognition of host-specific molecules results in a suppression of any immune response against that tissue, a process referred to as self-tolerance. A loss of self-tolerance leads to autoimmunity.
Using genetic mapping and other techniques, the team discovered there are actually two required mutations involved in the flies' apparent autoimmunity. While the first mutation kicked the immune response into overdrive, the other disabled the Suzuki flies' ability to label and recognize its own tissues.
One way the fly distinguishes its own tissues from those of pathogens is through the specific sugar molecules that are stamped on the layer of proteins coating internal tissues, scientifically referred to as glycosylated extracellular matrix proteins, Mortimer explained.
"We found the mutant flies fail to glycosylate proteins on their fat body extracellular matrix, and this causes blood cells of immune-activated flies to attack the fat body," Schlenke said.
Altered patterns of protein glycosylation have also been connected to autoimmune diseases like lupus in mouse models and human patients.
"We've figured out the general mechanism behind autoimmunity in these mutant flies, but there's still a long road ahead," Mortimer said. "Given that extracellular matrix proteins are broadly conserved across animals, it wouldn't surprise me if humans have a similar mechanism of self-tolerance. It would be great to explore this through additional research to see if mis-glycosylated extracellular matrix proteins explain mammalian autoimmune diseases."
More information: Nathan T. Mortimer et al, Extracellular matrix protein N-glycosylation mediates immune self-tolerance in Drosophila melanogaster, Proceedings of the National Academy of Sciences (2021). DOI: 10.1073/pnas.2017460118