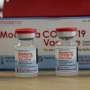
Moderna asks FDA to OK its booster shot for all adults
(HealthDay)—Moderna asked the U.S. Food and Drug Administration on Wednesday to allow emergency use of the company's coronavirus vaccine booster for all adults aged 18 and older.
Currently, the booster is approved for people 65 and older, along with adults in long-term care homes, those with underlying medical conditions and those with jobs that put them at high risk of exposure, the company said in a news release. The booster is given six months after the second shot of the two-dose initial vaccination series.
Moderna's booster is half the dose of the initial vaccination series: 50 micrograms vs. 100 micrograms.
New York City and some states aren't waiting for federal approval and have already made Moderna booster shots available to all adults.
The Moderna vaccine has been shown to be safe overall, but is associated with a small but increased risk of heart inflammation (myocarditis), particularly in young men.
"These cases are typically mild," Moderna Chief Medical Officer Dr. Paul Burton said at a news briefing earlier this month, NBC News reported. "While we don't fully understand the reasons and the etiology behind this, we think that it may be driven by testosterone."
He noted that no cases of myocarditis in young men have been reported in clinical trials of the booster doses, and added that the benefits of vaccination far outweigh any risks.
The FDA is expected to soon give the green light to Pfizer's COVID-19 vaccine booster shot for all adults, and it will be discussed at a U.S. Centers for Disease Control and Prevention advisory panel meeting scheduled for Friday.
Some experts still question the need for a booster among younger adults.
"By and large, healthy, younger people who have been vaccinated are handling breakthrough infections well," Dr. Jesse Goodman, a former chief scientist with the FDA and a professor of medicine and infectious diseases at Georgetown Medical Center in Washington, D.C.
"People have to realize giving a third dose of vaccine to healthy young people is not going to affect the pandemic," he told NBC News. "The way we're going to do that is by getting unvaccinated people immunized."
More information: Visit the U.S. Food and Drug Administration for more on COVID vaccines.
Copyright © 2021 HealthDay. All rights reserved.