This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new type of RNA could enhance vaccines and cancer treatments
It all started in the lab. Two Boston University doctoral students, Joshua McGee and Jack Kirsch, were creating and testing different types of RNA—strands of ribonucleic acid, built from chains of chemical compounds called nucleotides that help carry out genetic instructions in cells. They were determined to see whether RNA sequences crafted with small changes to their nucleotides can still work. After running dozens of experiments, they hit a dead end.
"At first, it was a failure," McGee says.
Decades of research have uncovered the mysteries of RNA in living cells. Without it, our cells couldn't perform fundamental tasks, like constructing other cells, carrying amino acids from one part of the cell to the other, or mounting immune responses to viruses.
But, more recently, scientists have figured out how to harness RNA to make treatments aimed at fighting genetic diseases and cancer. They've also learned how to use messenger RNA (mRNA) to make COVID-19 vaccines. The experiments that McGee and Kirsch perform are aimed at using RNA to deliver lifesaving drugs and create more effective vaccines than we have today.
Working alongside Mark Grinstaff, BU's William Fairfield Warren Distinguished Professor of biomedical engineering and chemistry, and Wilson Wong, a College of Engineering associate professor of biomedical engineering, they started talking about what to do next—and what to do with the chemical components left over from the initial experiments.
They decided to focus on modifying the chemical structure of a lesser-known type of RNA, called self-amplifying RNA (saRNA), which is manufactured in the lab and replicates itself multiple times in a cell to produce a higher number of the proteins it's programmed to make.
The new method worked: Their modified saRNA was replicating itself in a petri dish.
"Our reaction to that was a lot of excitement, but also the normal scientist thinking, 'Did we do this right?'" McGee says. "We went back to do it again and again. And we got the same results."
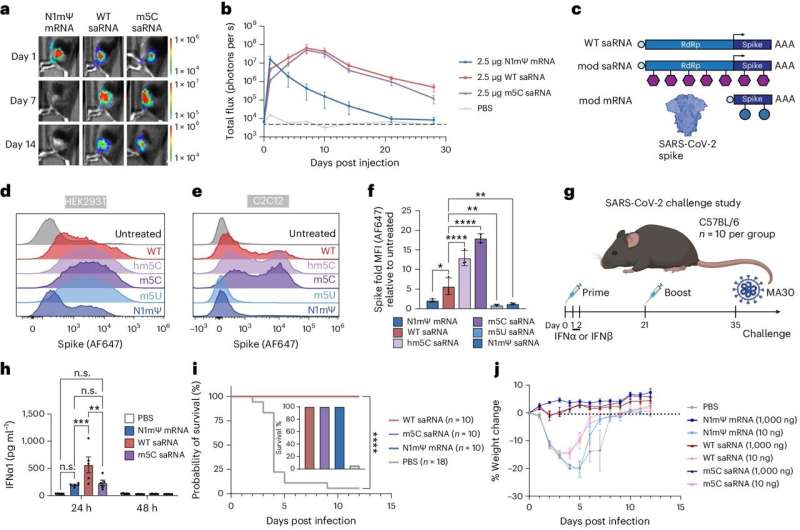
The results kicked off a yearlong research project that moved from Grinstaff's chemistry lab to Wong's genetics engineering lab to BU's National Emerging Infectious Diseases Laboratories (NEIDL), where they tested their modified saRNA as a vaccine against the COVID-19 virus. They found that a lower dose of their new vaccine in mice protected them from the disease just as well as current mRNA vaccines. Their findings are published in Nature Biotechnology.
It'll be years of further testing before this vaccine can be approved for humans. Even though there is one type of saRNA vaccine—approved last year for use in Japan—the researchers hope their modified version will make the technology more appealing to drug manufacturers, as well as overcome the challenges of using saRNA as a vaccine.
"The challenge with regular self-amplifying RNA is that there are two competing processes—the RNA is trying to make more and more protein, and at the same time the immune system is degrading it," says Grinstaff.
Standard mRNA COVID vaccines tell cells to produce a spike protein that mimics the real virus. That, in turn, causes the immune system to kick in and fight the virus. But an saRNA vaccine goes one step further by repeating those instructions to the cell over and over, making more of the machinery to create the spike proteins. More proteins means you don't need as high a dose and the immune system remembers how to fight the virus over a longer period of time.
"So the idea is that this could give you a long duration of protein expression, even when using a lower dose," Grinstaff says.
Another challenge is that saRNA could create a much-too-strong reaction that can lead to uncomfortable side effects—worse than those of current COVID vaccines, which typically cause some people to develop a mild fever or aches.
Grinstaff, Wong, and the team collaborated closely with Florian Douam, a BU Chobanian & Avedisian School of Medicine assistant professor of virology, immunology, and microbiology and a core faculty member at NEIDL. He and his team performed a study—called a "viral challenge"—to evaluate whether a COVID-19 vaccine built with the modified saRNA technology could protect mice more effectively against severe COVID-19 disease than earlier saRNA and mRNA vaccines.
"The viral challenge aspect was particularly important," Douam says. "It exposed how a very low dose of this novel saRNA technology is able to protect mice against lethal disease much more effectively than traditional saRNA and mRNA COVID-19 vaccines at a similar dose." Douam says that the new vaccine, which incorporates modified nucleotides called m5C (5-methylcytidine), also triggered very low levels of inflammation upon vaccination comparable to mRNA vaccines.
"There is still plenty of work to be done to unveil all of the advantages of this technology over other existing RNA vaccine approaches," Douam says. But this was a promising start.
The next question is whether their modified saRNA can provide longer-lasting protection against virus infection compared to existing RNA-based vaccines at a similar dosage.
More promising treatments
Besides COVID vaccines, the team's well-tolerated saRNA could open the door for other types of treatments and gene therapy.
"At the end of the day, this is a protein-producing system," Wong says, "a gene-delivery system."
For a genetic disorder, saRNA could be programmed to produce a missing gene or replace a defective one, Wong says. For treating lung, breast, and other cancers, "we can have it produce an anticancer drug for disease that requires a high dose and a lot of protein being made."
"That's why we're really excited about our self-amplifying RNA technology—because we think we can lower the dose that's needed to enable some of these therapeutic applications," Wong says. "That's how we envision it."
"There's so much work that we're doing now to further understand what we have discovered," says McGee, who is co-advised by Wong and Grinstaff. "There are a lot of publications out there that suggested research on saRNA would also fail. This made me realize that it's okay to try things that other people think might fail, because, who knows, they could be wrong."
More information: Joshua E. McGee et al, Complete substitution with modified nucleotides in self-amplifying RNA suppresses the interferon response and increases potency, Nature Biotechnology (2024). DOI: 10.1038/s41587-024-02306-z