A step towards the development of precision medicine against drug-resistant cancers
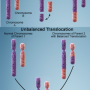
The generation of chimeric RNAs had been identified as an exclusive feature of cancer cells, with chimeric RNAs being recognized as biomarkers and drug targets for different cancers. Recent studies, however, revealed the presence of chimeric RNAs in normal cells. At present, the functional and evolutionary significance of chimeric RNAs are not well understood. Researchers from the Azrieli Faculty of Medicine of Bar-Ilan University in Israel have now proposed a hypothesis to explain the evolutionary and functional significance of chimeric RNAs in human cells. According to this hypothesis, chimeric RNAs are important drivers of phenotypic diversity in human cells.
In a new paper published in the journal Trends in Genetics, Dr. Milana Frenkel-Morgenstern and her postdoctoral student Dr. Sumit Mukherjee explained how the appearance of chimeric RNAs could shape the evolution of cancer cells. Furthermore, they demonstrated how chimeric RNAs could act as functional precursors of a gene and contribute to the origin of new genes and functional evolution during generation of new species.
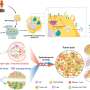
In another paper recently published by the same team in the journal Cancers, the researchers demonstrated how the appearance of chimeric RNAs cells could provide functional benefit for the adaptive evolution of cancer cells. Cancer progression can be explained by a two-phase evolutionary model whereby Phase I encompasses the evolution of cancer cells from normal cells, leading to the formation of tumors (i.e., macro-evolution), while in Phase II, evolution within tumors or heterogeneity (i.e., micro-evolution) occurs. This publication provided detailed insight into how chimeric RNAs could contribute to both phases of cancer evolution.
In addition, in 2012, the Dr. Frenkel-Morgenstern group identified a new class of chimeric RNA named sense-antisense (SAS) chimeras. These SAS chimeras are produced upon the joining of exons/introns from both sense and antisense transcripts of the same gene. However, their expression in human cells and their potential function, not yet known. In a recent paper published in NAR Genomics and Bioinformatics, they found SAS chimeras are prevalently expressed in both normal and cancer cells. Further, structural analysis of these SAS chimeras revealed that they could generate double-stranded RNA (dsRNA) structures and possibly play a role in regulating gene expression. Their study also identified five SAS chimeras within RNA-seq data of PCAWG (ICGC/TCGA) breast cancer samples and the MCF-7 breast cancer cell line, which were absent in healthy breast tissue and the MCF12A normal breast cell line. These findings suggest that the generation of various SAS chimeras could depend on cancer-related stresses unique to the individual and which are important for cancer evolution.
"Cancer heterogeneity is important because it provides cancer cells with the fitness to adapt to multiple stress conditions. Chimeric RNAs specific to a cancer sample are essential for cancer heterogeneity and also promote drug-resistant cancer evolution. This is also a potentially important reason for the failure of current cancer therapies. Consequently, the identification and characterization of cryptic hidden reservoirs of chimeric RNAs in cancer transcriptomes could reveal novel targets in designing personalized treatment of cancers," says Dr. Milana Frenkel-Morgenstern, of the Azrieli Faculty of Medicine of Bar-Ilan University, who led these studies.
The researchers plan to develop a liquid biopsy-based platform and determine whether this approach can accurately identify chimeric RNAs from patients' cell-free DNA (cfDNA) and cell-free RNA (cfRNA). This would facilitate tracking the origin of certain chimeric RNAs during different phases of cancer treatment, which will help in the design of personalized medicine.
These studies were partially supported by a Kamin grant from the Israel Innovation Authority and a PBC fellowship for outstanding postdoctoral researchers (to Dr. Sumit Mukherjee, who is the first author on the three papers described) from the Israel Council for Higher Education.
More information: Sumit Mukherjee et al, Evolutionary impact of chimeric RNAs on generating phenotypic plasticity in human cells, Trends in Genetics (2021). DOI: 10.1016/j.tig.2021.08.015
Sumit Mukherjee et al, Emerging Role of Chimeric RNAs in Cell Plasticity and Adaptive Evolution of Cancer Cells, Cancers (2021). DOI: 10.3390/cancers13174328
Sumit Mukherjee et al, Computational analysis of sense-antisense chimeric transcripts reveals their potential regulatory features and the landscape of expression in human cells, NAR Genomics and Bioinformatics (2021). DOI: 10.1093/nargab/lqab074