Revolutionary 3D imaging maps how breast cancer spreads
WEHI researchers have developed enhanced imaging technology that can model how breast cancer cells invade and spread into bone and remodify themselves to fuel tumor growth.
The team found that tumor spread occurred at specific locations in the bone, not randomly as previously thought. They also showed that breast cancer cells 'renovate' the bone to create an environment that fuels their spread, while starving the body of essential nutrients.
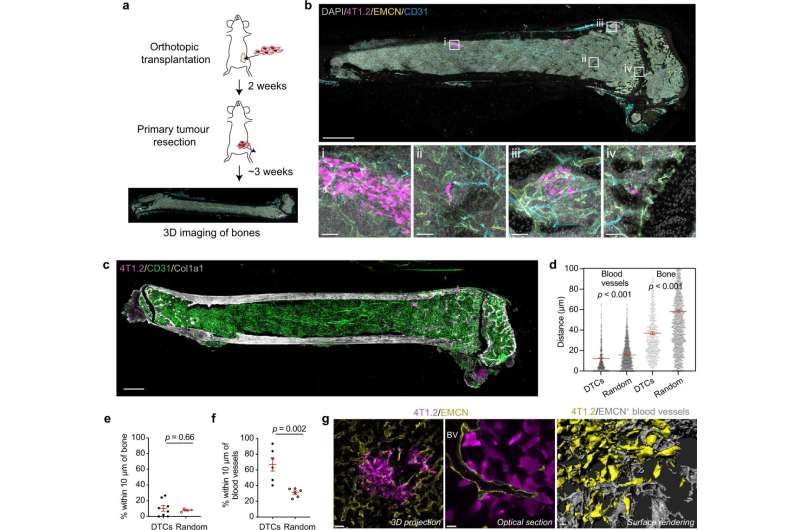
Using WEHI's Centre for Dynamic Imaging, the research team took hundreds of images to create three-dimensional (3D) models of the bone marrow and the blood vessels that run through them—some as tiny as a fraction of a micron. These images were used to better understand why breast cancer cells metastasise (spread) into bone, and what factors facilitate their growth.
The research was led by Dr. Raymond Yip, Associate Professor Edwin Hawkins, Professor Geoff Lindeman and Professor Jane Visvader, with colleagues at WEHI. It was published in Nature Communications.
Radical renovation
Bone marrow is one of the most common sites of metastasis in people with breast cancer. Spread of cancers to secondary organs, such as the bone, is often incurable, and breast cancer patients with bone metastases typically have a very poor prognosis.
Dr. Yip said the research provided a fascinating insight into how cancer cells colonize the bone marrow, by mapping out the vessels and the tumor structures next to them.
"We think of bones as these static, structural organs—but they're highly dynamic," he said.
"Our research shows breast cancer cells preferentially reside near a specific blood vessel subtype in the bone called the type H vessels. In other words, breast tumor cells selectively home to a specialized vasculature, suggesting type H vessels are supplying certain growth factors to nurture breast cancer cells growth in bone."
Associate Professor Hawkins said the idea that cancer cells can remodel their environment isn't new, however it hadn't been explored in a "tricky organ" like bone marrow until now.
"What Raymond showed us through his incredible 3D images is that a tumor cell will move to the bone marrow and completely renovate that home" Associate Professor Hawkins said.
"We saw that when breast cancer cells metastasise to the bone marrow, they release tumor-derived growth factors that enable them to 'remodel' to create a favorable environment that further facilitates their growth—unfortunately at the detriment of the whole body."
By delving into the symbiotic relationship between our host bodies and these tumor cells, the researchers hope their discoveries can one day lead to a better understanding of mechanisms that these cells work with that can make them easier to treat or prevent tumor spread.
Cancer treatment enhancements
While breast cancer patients are the focus of the current research, spread of tumors to the bone is common in other cancers, including prostate, lung, kidney and thyroid cancers and melanoma.
Professor Visvader said these mechanisms could be a target for future therapeutic discovery.
"Cancer therapeutic discovery has expanded considerably over past decades to not only target the cancer cells directly, but also the mechanisms used by cancer cells to enhance their growth," she said.
"It will be important to further understand the mechanisms by which tumor cell-derived factors remodel blood vessels, as this could help define new therapies for patients in the future. We also hope to take this innovative 3D imaging technique we have developed and extend it to other diseases that involve bone metastases."
More information: Raymond K. H. Yip et al, Mammary tumour cells remodel the bone marrow vascular microenvironment to support metastasis, Nature Communications (2021). DOI: 10.1038/s41467-021-26556-6