New precision technology for human glioma immunotherapy
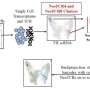
![Figure 1. Tumor surfaceome mapping (TS-MAP) of glioma tumors. (A) Schematic outline of procedures for uncovering the surfaceome and endocytome in intact patient, mouse tumors, primary GBM 2D and 3D-spheroid cultures (A1). (A2) A workflow was established for reversible biotinylation, TS enrichment by high-affinity HPLC, and LC-MS/MS, integrated with high-resolution imaging using a GaAsP array-confocal detector (Airyscan) and FACS. (A3) An in-house TS classifier (SURFME) was curated for filtering and categorization of bona fide membrane proteins, identifying potential target candidates for further validation by immunofluorescence analyses of matched tumor sections, and pilot ADC treatment experiments in vitro. (B) Quantification of surfaceome and endocytome in primary GBM cell cultures grown in 2D and 3D. Representative histograms from FACS analysis of nonbiotinylated (control), total surface biotinylation (surface), residual cell-surface signal following MesNa treatment (surface + MesNa), and endocytosed surface proteins (internalized) in U3065 (Upper) and U3082 (Lower) GBM cells, grown in 2D or 3D, as indicated. (C) Quantitative analysis of the experiment presented in B with endocytosed proteins expressed as a fraction of the total surfaceome protein abundance. Data are presented as mean ± SD from three independent experiments each performed in triplicates. MFI, median fluorescence intensity. (D) High-resolution Airyscan imaging of surface and endocytosed biotinylated proteins (green) in GBM cells grown in 2D or 3D (shown in representative brightfield images), as indicated. (Scale bars, 5 μm [U3065 2D], 2 μm [U3065 2D Inset], 50 μm [U3065 and U3082 3D, Left], and 20 μm [U3065 and U3082 3D, Left].) (E) Airyscan imaging of endocytosed biotinylated proteins (green) and the early endosome marker EEA1 (magenta) in GBM cells grown in 2D or 3D, as indicated, after 1.5 h of endocytosis. (Scale bars, 5 μm, and 2 μm for Insets.) (F) Airyscan imaging for visualization of endocytosed surface proteins in the membrane of endolysosomal vesicles, as indicated by CD63-mCherry (magenta). Images were captured 45 min after initiation of endocytosis (see also Movies S1 and S2). (Scale bars, 2 μm.) (D–F) Shown are representative images from at least three independent experiments. White squares indicate zoomed areas shown in Insets. White arrows indicate colocalization. Credit: DOI: 10.1073/pnas.2114456119 New precision technology for human glioma immunotherapy](https://scx1.b-cdn.net/csz/news/800a/2022/new-precision-technolo.jpg)
In recent years, great advances have been made in the development of new successful immunotherapies to treat cancer. CAR T-cell therapy and antibody treatments are two types of targeted immunotherapies that have revolutionized areas of cancer care. However, there are still significant challenges in the identification of cancer cell surface proteins as targets for immunotherapies. A research group at Lund University in Sweden is well on the way and have now published their findings in PNAS.
Immunotherapies have revolutionized the treatment of cancer and, in some cases, are able to cure patients with advanced disease. Immunotherapies with CAR T-cells and antibodies share a focus on specific target proteins expressed on the surface of tumor cells, known as cell surface tumor antigens.
"The great challenge is that the structure of cell surface tumor antigens differs between patients and between primary tumors and metastases. There is a great need both for new strategies and for high precision identification of accessible, treatable cell surface tumor antigens at a personalized level. We have worked for many years to establish new methods that provide knowledge about antigens on the surface of cancer cells as a target for immunotherapies," says Mattias Belting, professor of clinical oncology at Lund University and consultant at Skåne University Hospital.
Now, he and his research group at Lund University and Skåne University Hospital have developed a new precision medicine technology which makes it possible to carry out comprehensive mapping of the whole cell surface tumor antigen landscape in patients.
The method developed by the research team, Tumor Surfaceome Mapping, TS-MAP, makes it possible to carry out a direct analysis of all accessible cell surface tumor antigens in tumor tissue from patients.
In a close collaboration between neurosurgery, oncology and advanced proteomics in Lund, the researchers were able to identify several cell surface tumor antigens in fresh tissue from patients with aggressive brain tumors and for which there is currently no effective treatment.
"Our new findings with patient cells and tissues point to the fact that tumor cells fundamentally change their surface landscape when they are removed from their natural, three-dimensional environment, which is important information for future research in the area," says Mattias Belting. "The methods previously developed to identify cell surface antigens or to produce antibodies targeting tumor cells use two-dimensional models, which, according to our findings, misrepresent the situation in patient tumors."
An important advantage of the TS-MAP technology is that it provides a comprehensive image of the cell surface antigens displayed on the surface of the cancer cell, as well as information about the specific cell surface antigens that have a high ability to infiltrate cancer cells, and can destroy them from within.
"This is important given that the next generation of antibody-based drugs in oncology are built on the combination of a target-seeking antibody which recognizes the cell surface tumor antigen and a cytotoxin or radionuclide that has been linked to the antibody. These antibody-drug conjugates (ADCs) specifically target and kill cancer cells from within while healthy cells without the cell surface tumor antigen are spared," says Mattias Belting.
Mattias Belting says the results of the study clearly highlight the possibilities and need for personalized strategies based on the great repertoire and variation of tumor antigens in patient tumors. It is also significant that the analysis is carried out on intact tissue.
"Precision medicine in immunotherapy for the treatment of cancer is promising, but also very challenging. In addition to the variation of tumor antigen expressions between and within tumors, we still have insufficient knowledge about the interaction between cancer cells and immune cells in the tumor microenvironment. Currently, we talk about each individual patient needing to be matched to a drug. Perhaps it is the other way around, that we must design a specific drug to match the individual patient, however impossible that may sound," concludes Mattias Belting.
More information: Valeria Governa et al, Landscape of surfaceome and endocytome in human glioma is divergent and depends on cellular spatial organization, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2114456119