Researchers study how gene expression changes in the brain in Alzheimer's disease
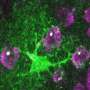
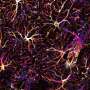
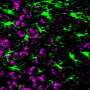
Although Alzheimer's disease is one of the most studied pathologies due to its high prevalence, the molecular changes that cause astrocytes, a type of brain cell, to become reactive astrocytes, manifesting a very pronounced morphological change in response to a stressful situation, are still unknown. Neither is it known why neurons in diseased brains have difficulty communicating with each other or with the astrocytes themselves.
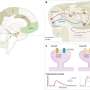
Now, in an article published in Neurobiology of Disease, an international group of researchers with expertise in these cells and the study of neurodegenerative diseases analyzed genetic data from post-mortem brain samples from nearly 800 individuals, to determine the differences between gene expression in astrocytes and neurons from brains with the disease and in cells from brains of people without a diagnosis of dementia, the control group. The samples came from the Alzheimer Disease Knowledge portal and were generated by three American clinics: Mount Sinai Hospital, the Mayo Clinic and the Religious Order Study/Memory and Aging Project.
Researchers studied the set of RNA molecules, or cellular transcriptome, which is used to determine which of all the genes are being expressed and to what extent. "By studying the transcriptome, we can see if there are silenced or overexpressed genes, and we can understand what is happening inside neurons and astrocytes," explains Elena Galea, researcher at the Institut de Neurociències (INc-UAB) and first author of the article.
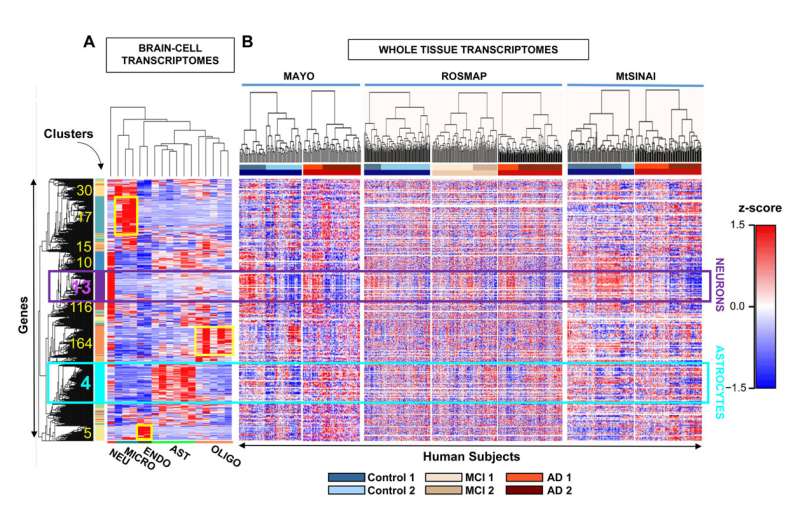
The results have shown a high genetic heterogeneity among people with the same clinical diagnosis and, also, that more than half of the control individuals have a molecular profile of Alzheimer's disease, which is characterized by decreased expression of synaptic genes due to neuronal damage and death. "This could indicate that these people were at a very early stage of the disease (still without symptoms) and would reinforce the idea that clinical diagnosis has to be complemented with the search for molecular markers, such as neuronal synapse proteins, to determine the phase in which the patient is," explains Lydia Giménez-Llort, author of the article and researcher at the Department of Psychiatry and Legal Medicine of the UAB and the INc-UAB. "In this sense, we are working together with the Pasqual Maragall Foundation to detect astrocyte proteins in the blood of patients with preclinical Alzheimer's disease," adds Dr. Galea.
The study also shows how, as the disease progresses, astrocytes decrease the expression of genes that code for mitochondrial proteins, which prevents the mitochondria of these cells (basic organelles for cellular energy) from functioning well. This effect could be an adaptation of the astrocytes to compensate for the toxicity of the amyloid protein and would be impairing communication between astrocytes and neurons. "We believe that this adaptation by astrocytes contributes to the worsening of the disease and could therefore be a key point in preventing its progression," explains Dr. Galea.
The study is the most complete transcriptomic analysis of human astrocytes in Alzheimer's disease to date and is of great relevance due to the number of samples analyzed. The results highlight the need to use molecular data to stratify patients into more genetically homogeneous groups for clinical trials and to obtain a more accurate diagnosis and treatment of the disease. Also, they open the door to developing targeted therapies to protect the function of astrocyte mitochondria.
More information: Elena Galea et al, Multi-transcriptomic analysis points to early organelle dysfunction in human astrocytes in Alzheimer's disease, Neurobiology of Disease (2022). DOI: 10.1016/j.nbd.2022.105655