March 17, 2022 report
Gene therapy for hemophilia A shows promise in phase 3 clinical trial
An international team of researchers has found more evidence that a new gene therapy may help some people with hemophilia A. In their paper published in the New England Journal of Medicine, the group describes the characteristics of the clinical trial and provides data regarding the effectiveness of the therapy. Courtney Thornburg, with the Hemophilia and Thrombosis Treatment Center, Rady Children's Hospital San Diego, has published an editorial piece in the same journal issue outlining the history of gene therapy development for hemophilia A and the work done by the team in this new effort.
Hemophilia A is a genetic disease that impacts the production of a protein called factor VIII—those who have the disease experience blood clotting issues. It more typically impacts male patients because it involves problems with an X chromosome. Hemophilia A is classified into three categories: mild, moderate and severe. In this new effort, the researchers trialed a treatment for male hemophiliac patients with severe symptoms.
Currently, patients with hemophilia A are treated with infusions of factor VIII, and while it is effective at allowing patients to live normal lives, the treatment is considered to be burdensome and expensive. For that reason, researchers have been studying gene therapy. Gene therapy for hemophilia has been pursued for over 20 years, but factor VIII presents more challenges than factor IX (associated with hemophilia B)—this gene therapy, like most others of its kind, involves injecting an adeno-associated virus into a patient, which initiates gene transfer, thereby correcting the problem at its roots.
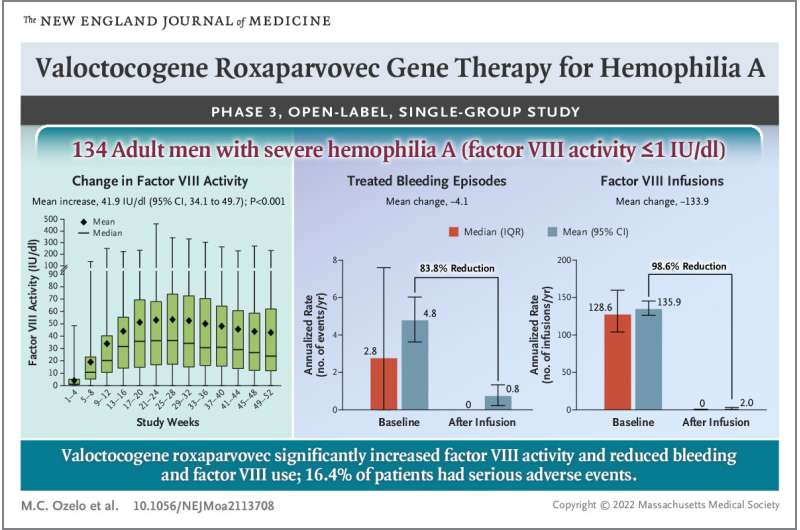
In the clinical trial, 132 men with hemophilia A who were otherwise healthy were given a one-time infusion of valoctocogene roxaparvovec, and were monitored for just over a year. The researchers found that almost all of the patients saw some increase in factor VIII levels—but not equally. After one year, 88% of them had levels high enough to classify them as having mild or no form of the disease. Unfortunately, there were also a wide variety of side effects experienced by all of the patients. Most concerning were elevated levels of liver enzymes that suggest inflammation, possibly leading to damage to the liver. Also, levels of factor VIII leveled off after one year and began to drop in the second. It is still not known how far they will drop in subsequent years.
More information: Margareth C. Ozelo et al, Valoctocogene Roxaparvovec Gene Therapy for Hemophilia A, New England Journal of Medicine (2022). DOI: 10.1056/NEJMoa2113708
Courtney D. Thornburg, Prepare the Way for Hemophilia A Gene Therapy, New England Journal of Medicine (2022). DOI: 10.1056/NEJMe2200878
© 2022 Science X Network