3D optical imaging of the human pancreas in disease
Max Hahn's doctoral thesis characterizes insulin producing β-cell dynamics of streptozotocin-induced diabetes in mice. Novel approaches were also developed that define human pancreatic structure-function relationships at the whole-organ level, as well as using autofluorescence as a 3D tool for pancreatic related pathologies, such as cancer and diabetes.
Diabetes mellitus is a disease that globally affects >400 million individuals. As such, understanding pancreatic disease-related mechanisms is important in developing new and more effective therapies. The deep abdominal location of the pancreas and the relatively low resolution of current clinical non-invasive imaging approaches render pancreatic islets difficult to study when visually assessing endocrine function.
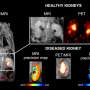
However, post-mortem studies of the pancreas and rodent disease models offer unique insights into the mechanisms underlying diabetes disease dynamics. Mesoscopic 3D imaging has proven to be a reliable technique in quantifying cellular/anatomical features of the mouse pancreas. However, the technique is rarely applied to human tissues, including the whole pancreas.
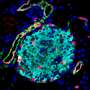
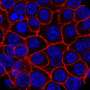
A modified approach was developed to 3D image a whole human organ. Specifically, pancreata from non-diabetic and type 2 diabetic (T2D) donors, analyzing over 200,000 islets, revealed many new features, including high islet dense regions and intra-islet hemorrhaging. When applied to a longstanding type 1 diabetic (T1D) pancreas, ~173,000 insulin-positive objects were identified.
These data indicated several important regional differences in β-cell mass, such as the head showing the highest density, which may reflect key aspects of disease dynamics. Furthermore, discrete regions were identified with a "punctuated distribution" of single β-cells in close proximity of each other. Elucidating the nature of these clusters may provide important cues for the future development of clinical interventions that promote tissue regeneration.
Altogether, this study represents the first whole organ characterization of β-cell distribution at high resolution. As such, it may serve as an important advancement towards detailed whole organ analyses of endocrine cell identity/function, via a wide range of markers, in the study of normal anatomy and pathophysiology of the human pancreas, including the study of general (normal) pancreas anatomy (e.g. during development, but also the aging pancreas) and the diseased pancreas (e.g. in diabetes and pancreatic cancer). As such, these contributions may help to better understand pancreatic pathophysiology with high level of spatial and molecular detail, at the level of the whole organ.
New methods for mesoscopic 3D imaging (optical projection tomography and light sheet fluorescence microscopy) were developed. These include new antibody labeling techniques, use of autofluorescence for outlining tumor margins in human pancreatic cancer, and pipelines for 3D image analysis and whole organ assembly.
More information: Doctoral thesis abstract: umu.diva-portal.org/smash/reco … anguage=en&pid=diva2%3A1649580&dswid=-7978