Newly discovered mechanism points to cause of drug-induced parkinsonism
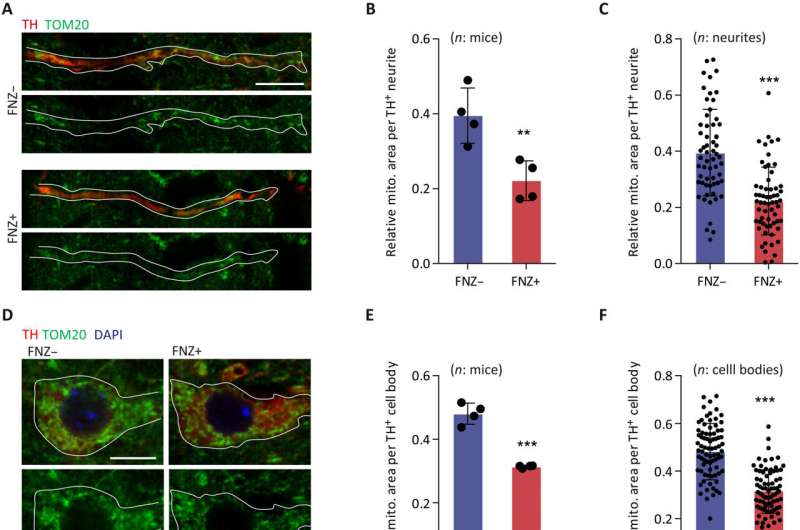
Drug-induced parkinsonism is not well understood. Chronic use of flunarizine (FNZ), a drug often used for treating dizziness, migraine, epilepsy, and peripheral vascular diseases, may induce parkinsonism. Previous investigations point out that the disruption of mitochondrial quality control, essential for mitochondrial homeostasis and function, degrades brain functions, and is causally related to parkinsonism disease. Still, there is limited knowledge about the exact mechanism of drug-induced parkinsonism, which hinders the development of prevention and treatment.
Recently, researchers from Guangzhou Institutes of Biomedicine and Health (GIBH) of the Chinese Academy of Sciences (CAS) and the Second Affiliated Hospital of Zhejiang University School of Medicine have revealed the mechanisms of FNZ-inducing parkinsonism by provoking the integration of mitochondria and lysosomes, named as mitolysosome, and causing mitochondrial reduction in brains. This study was published in Science Advances.
The researchers noticed that mice treated with FNZ showed parkinsonism-like symptoms in the rotarod test, the open-field test and Morris water maze test, including decreased coordination and balance and decreased ability to learn and to memorize.
The team detected an increase in glucose uptake in the brains of FNZ-treated mice by [18F]-fluoro-2-deoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) scanning, and significant reductions of mitochondrial proteins specifically in brains, but not in other tissues.
Looking into the mechanism of mitochondrial elimination, the researchers uncovered that FNZ-induced mitochondrial direct entry into lysosomes, formatted mitolysosome, a new organelle structure, which then mediated a VAMP2/STX4-dependent exocytosis, leading to the reduction of the amount of mitochondria. The process was canonical or noncanonical mitophagy independent. Furthermore, a genome-wide CRISPR/Cas9 knockout screening was employed to identify genes that were necessary for mitochondrial elimination. Results indicated that lysosome-associated mitochondrial exocytosis may contribute to parkinsonism.
This study showed a FNZ-based method for total depletion of mitochondria, which can be used to explore the novel function of mitochondria or to replace the mitochondrial DNA mutations of patients with functional mitochondria.
More information: Feixiang Bao et al, Mitolysosome exocytosis, a mitophagy-independent mitochondrial quality control in flunarizine-induced parkinsonism-like symptoms, Science Advances (2022). DOI: 10.1126/sciadv.abk2376





















