Investigational COVID mucosal vaccine protects against disease and transmission
In animal studies that mimic human exposures, an investigational COVID vaccine designed to be taken orally not only protects the host, but also decreases the airborne spread of the virus to other close contacts.
The study, led by Duke researcher Stephanie N. Langel, Ph.D., demonstrated the potential of a COVID vaccine that works through the mucosal tissue to neutralize the SARS-CoV-2 virus, limiting infections and the spread of active virus in airborne particles.
The findings are published today in the journal Science Translational Medicine.
"Considering most of the world is under-immunized—and this is especially true of children—the possibility that a vaccinated person with a breakthrough infection can spread COVID to unimmunized family or community members poses a public health risk," Langel said. "There would be a substantial benefit to develop vaccines that not only protect against disease, but also reduce transmission to unvaccinated people."
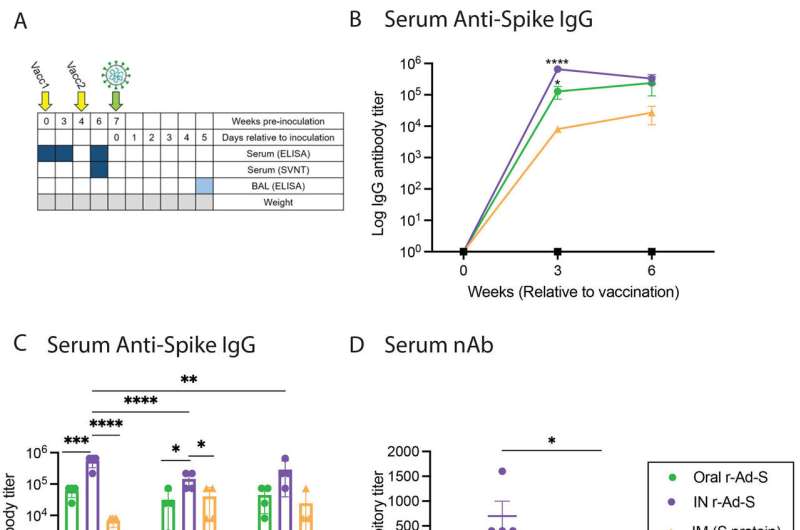
Langel and colleagues—including teams from the vaccine developer Vaxart, and a clinical research non-profit, Lovelace Biomedical Research Institute—tested a vaccine candidate that uses an adenovirus as a vector to express the spike protein of the SARS-CoV-2 virus. The human vaccine is designed to be taken as a pill.
In studies using hamsters, the vaccine elicited a robust antibody response in blood and the lungs. When the animals were exposed to the SARS-CoV-2 virus at high levels, prompting breakthrough infections, they were less symptomatic than non-vaccinated hamsters, had lower amounts of infectious virus in the nose and lungs. Because of this, they did not shed as much virus through normal airborne exposures.
Unlike vaccines that are injected into the muscle, Langel said, mucosal immunizations increase production of immunoglobulin A (IgA)—the immune system's first line of defense against pathogens—in the nose and lungs. These mucosal ports of entry are then protected, making it less likely that those who are vaccinated will transmit infectious virus during a sneeze or cough.
"Our data demonstrate that mucosal immunization is a viable strategy to decrease the spread of COVID through airborne transmission," Langel said.
Langel said the study focused on the original SARS-CoV-2 virus, and new studies will be designed to test the vaccine against Omicron variants.
In addition to Langel, study authors include Susan Johnson, Clarissa I. Martinez, Sarah N. Tedjakusuma, Nadine Peinovich, Emery G. Dora, Philip J. Kuehl, Hammad Irshad, Edward G. Barrett, Adam Werts, and Sean N Tucker.
More information: Stephanie N. Langel et al, Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model, Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.abn6868