The role of the cerebellum in absence seizures
Stimulation of certain cerebellar areas could help combat absence seizures. However, what happens at the cellular and molecular level in the brain in this form of epilepsy and how exactly stimulation has an effect is not yet understood in detail. Researchers at Ruhr-Universität Bochum (RUB) have gained new insights by conducting experiments with mice. The team led by Dr. Jan Claudius Schwitalla and Professor Melanie Mark from the RUB Behavioral Neuroscience research group describes the results in the journal Cellular and Molecular Life Sciences from 19 March 2022. They cooperated with the Erasmus Medical Center in Rotterdam and Utrecht as well as with colleagues from Bonn, Münster and München.
Abrupt loss of consciousness
More than 1.5 million people worldwide suffer from absence seizures, also known as petit mal seizures. Patients experience an abrupt loss of consciousness and lapse into a paralysis of behavior that lasts for a few seconds. Absence seizures often occur in children between the ages of four and twelve and are often mistaken for daydreaming. They are linked to altered brain activity, which is visible in brain activity recordings as so-called spike-and-wave discharges (SWDs). The characteristic activity pattern originates from the rhythmic and synchronized activity of nerve cells in the cerebral cortex and thalamus.
Since the nuclei located deep in the cerebellum have a widespread connectivity to various regions of the brain, researchers proposed that it might be possible to treat seizures by stimulating the cerebellar nuclei. Experiments with rodents by other research groups showed that such stimulation can indeed stop absence seizures. However, it is unclear what underlies this effect at the cellular and molecular level.
Cerebellar stimulation against abnormal brain activity
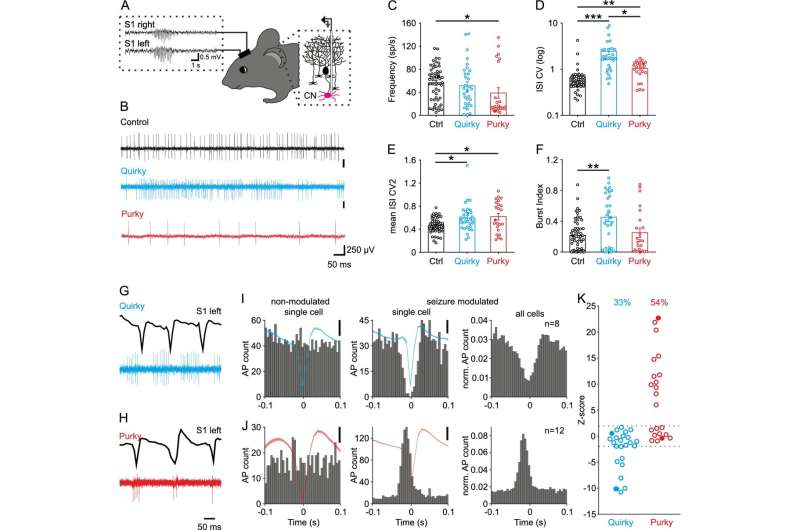
The Bochum-based researchers worked with mice that develop absence seizures due to a lack of the P/Q-type calcium channel in nerve cells of the cerebellum. They found that cells of the cerebellar nuclei were firing abnormally, and that stimulation of these cells could prevent further SWDs. Therefore, they stimulated the cerebellar nuclei by administering a pharmacological substance or via chemogenetic stimulation. For chemogenetic stimulation, a genetically modified receptor is introduced into cells so that they can be activated by a specifically designed molecule normally not present in the brain. This allowed the researchers to slowly increase the activity of the cerebellar nuclei cells and thus prevent the occurrence of further SWDs in mice.
Furthermore, the team used optogenetic stimulation to briefly increase the activity of cells in the cerebellar nuclei and to stop on-going SWDs after they have started. This technique uses proteins from algae that can be turned on by light to increase the activity of nerve cells. Overall, the study confirmed that targeted stimulation of the cerebellar nuclei could become a therapeutic approach for people suffering from absence seizures.
More information: Jan Claudius Schwitalla et al, Controlling absence seizures from the cerebellar nuclei via activation of the Gq signaling pathway, Cellular and Molecular Life Sciences (2022). DOI: 10.1007/s00018-022-04221-5