New drug candidate developed to treat type 2 diabetes
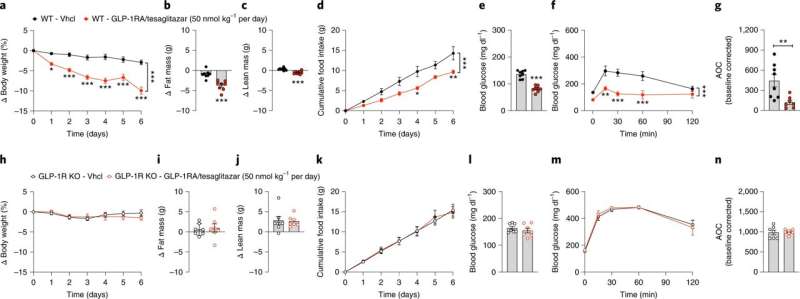
A team of researchers from Helmholtz Munich, the German Center for Diabetes Research (DZD) and Novo Nordisk have developed a new hormone combination for the future treatment of type 2 diabetes. The scientists have combined the blood sugar-reducing effects of the drugs tesaglitazar and GLP-1 (Glucagon-like peptide-1) in a new and highly effective drug. The advantage is that, by combining tesaglitazar with GLP-1, the tesaglitazar only enters tissue that contains GLP-1 receptors. This reduces the adverse effects of tesaglitazar while increasing the effects on sugar metabolism. The new drug has already been successfully tested in animal studies. The findings were published in Nature Metabolism.
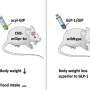
The drug tesaglitazar improves glucose and fat metabolism in patients with type 2 diabetes. It acts on two receptors within the cell nucleus to increase insulin sensitivity. This was proven in phase 3 clinical trials. However, tesaglitazar also caused unwanted effects, such as signs of kidney damage. Nevertheless, in order to use the drug therapeutically, the researchers used a trick: They biochemically combined tesaglitazar with the gastrointestinal hormone GLP-1, which since several years successfully used to treat type 2 diabetes. This allows the combined drug to only act on cells and tissue that contain GLP-1 receptors.
"This trick enabled us to combine the blood sugar-reducing effects of GLP-1 and tesaglitazar into a single highly effective molecule, while keeping tesaglitazar away from tissues that it could damage," explains PD Dr. Timo Müller, corresponding author, director of the Institute of Diabetes and Obesity, and scientist at DZD.
The new drug improves glucose tolerance and sugar metabolism
The new drug has already been successfully tested in animal studies: "The sugar metabolism of obese and diabetic male mice improved to a far greater extent compared with treatment using only the GLP-1 hormone or tesaglitazar alone—and with no damaging adverse effects to the liver or kidney," says Prof. Kerstin Stemmer, one of the lead authors of the study.
The substance was particularly effective in increasing glucose tolerance levels. Only minimal doses of the new drug were required to achieve sustainable improvement of glucose metabolism. "This drug has great potential for the acute treatment of elevated blood sugar levels associated with type 2 diabetes," says Aaron Novikoff, another lead author of the study.
The researchers now want to investigate whether this drug also has the potential to treat humans with type 2 diabetes and whether the efficacy of this new combination therapy can be optimized further using biochemical modifications.
The team comprising Prof. Dr. Matthias Tschöp (scientific director of Helmholtz Munich), PD Dr. Timo Müller, Richard DiMarchi, Ph.D. (Indiana University), and Dr. Brian Finan (Novo Nordisk) has been working for many years on new drug candidates for the treatment of type 2 diabetes and obesity. Poly-agonists simultaneously mimic the effects of various hormones. Clinical trials have shown that some poly-agonists are exceptionally promising in the treatment of obesity and type 2 diabetes and are already in phase 2 and phase 3 trials. This year, in the U.S., the first dual agonist drug for the treatment of type 2 diabetes was approved. The drug combines GLP-1 and GIP (glucose-dependent insulinotropic polypeptide) receptor agonists that mimic the effects of the intestinal hormones GLP-1 and GIP.
More information: Carmelo Quarta et al, GLP-1-mediated delivery of tesaglitazar improves obesity and glucose metabolism in male mice, Nature Metabolism (2022). DOI: 10.1038/s42255-022-00617-6