Protein transformation drives cancer development
A change in function in a mitochondrial antioxidant protein increases stem cell gene expression that promotes the development of more aggressive cancerous cells, according to a recent Northwestern Medicine study published in Proceedings of the National Academy of Sciences.
While the protein superoxide dismutase-2 (SOD2) has been known to scientists as both a mitochondrial antioxidant, a function that usually protects against the development of cancer, and as a tumor promoting agent later in established disease, the specific mechanisms underlying these dual behaviors have not been fully understood.
"We provided the discovery of a new form of this enzyme that is substantially different than the antioxidant that is involved in genomic reprogramming promoting the evolution of these more aggressive forms of breast cancer. Although we focused on breast cancer, we believe this finding could also be generalized to other forms of cancer, as well," said Marcelo Bonini, Ph.D., professor of Medicine in the Division of Hematology and Oncology and senior author of the study.
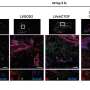
In the current study, the authors explored the biological function of SOD2, especially when it was located in a cell's nucleus. Using a variety of techniques including cell cultures, immunofluorescence microscopy, an interactome assay, and RNA sequencing analysis, Bonini and his collaborators observed that a cellular process called acetylation led to SOD2 increasing its affinity to binding iron, instead of manganese, and caused it to localize in the nucleus instead of mitochondria.
The post-acetylated form of the protein, which targeted the nucleus—called NLS-SOD2—was found to promote stem cell gene expression, specifically by removing epigenetic marks that usually suppress cancerous cells from activating stem cell programs. The authors also observed that the cells which expressed NLS-SOD2 were more likely to acquire a greater potential to evolve into more aggressive forms of cancer.
"We think that the presence of the acetylated form of SOD2 is a molecular signature of breast cancer cases that are more likely to recur. Therefore, acetylated SOD2 may be developed into a biomarker of forms of the disease that must be treated more aggressively. It is also possible that targeting, nuclear-localized SOD2 pharmacologically will provide novel therapeutics to suppress cancer stem cells that are resistant to treatment and metastatic," Bonini said.
The localization in the nucleus of cancer cells, as well as unique structural features granted by acetylation, suggest that targeting NLS-SOD2 may hold potential for creating diagnostic tools or potential future treatments, according to the authors.
More information: Diego R. Coelho et al, Nuclear-localized, iron-bound superoxide dismutase-2 antagonizes epithelial lineage programs to promote stemness of breast cancer cells via a histone demethylase activity, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2110348119