52% and 66% death reduction for molnupiravir and nirmatrelvir–ritonavir users among inpatients with COVID-19
A research team including members from the Department of Pharmacology and Pharmacy, School of Public Health, Department of Family Medicine and Primary Care, School of Clinical Medicine, LKS Faculty of Medicine, The University of Hong Kong (HKUMed), and Laboratory of Data Discovery for Health (D²4H) have conducted one of the first real-world studies to explore the inpatient use of oral antivirals during a pandemic wave dominated by the SARS-CoV-2 omicron variant.
Initiation of novel oral antiviral treatments in hospitalized patients with mild-to-moderate COVID-19 not requiring oxygen therapy on admission showed substantial clinical benefit to reduce mortality, disease progression, and achieve lower viral burden. The findings are now published online in the journal The Lancet Infectious Diseases.
Background
Use of oral antivirals in patients with COVID-19 may lower their risks of hospitalization and death and reduce the burden on health care systems.
Clinical trials observed no clinical benefits with molnupiravir use in the inpatient setting among patients with moderate-to-severe COVID-19, and reported reductions in the hospitalization or death with oral antivirals use (30% for molnupiravir and 88% for nirmatrelvir–ritonavir respectively) in non-hospitalized patients with mild-to-moderate COVID-19.
However, real-world evidence of oral antiviral use in patients infected with the SARS-CoV-2 omicron variant is insufficient.
Research findings
The study analyzed data from a territory-wide retrospective cohort of patients in Hong Kong who were hospitalized with a confirmed diagnosis of SARS-CoV-2 infection between 26 February and 26 April 2022. Among 40,776 patients hospitalized with SARS-CoV-2 infection during the study period, we selected patients who were eligible to receive oral antivirals.
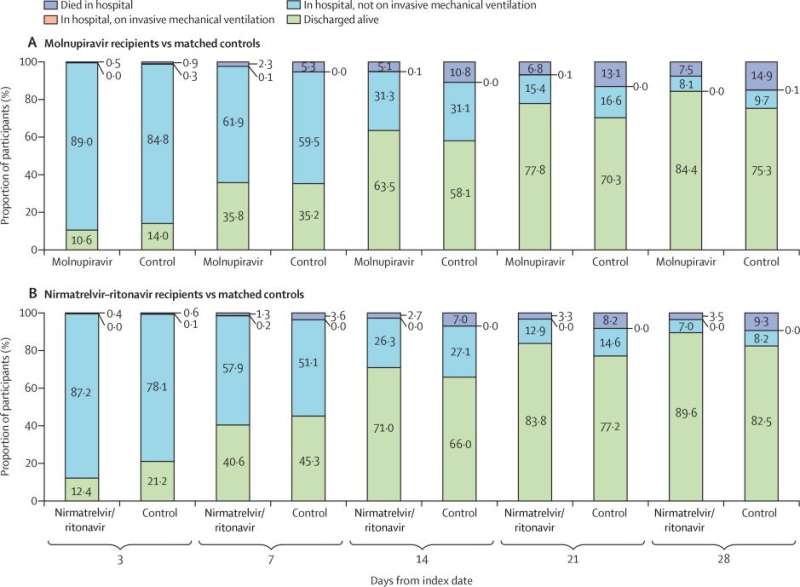
This analysis included 1,856 early molnupiravir recipients (i.e. receiving molnupiravir within the first two days of admission) and 1,856 matched controls, and 890 early nirmatrelvir-ritonavir recipients and 890 matched controls.
A lower risk of all-cause mortality was observed in molnupiravir recipients versus matched controls and in nirmatrelvir–ritonavir recipients versus matched controls. Oral antiviral recipients also had lower risks of the composite disease progression outcome and need for oxygen therapy than controls. Time to achieving a low viral burden was significantly shorter among oral antiviral recipients than matched controls.
Significance of the study
"Current guidelines are now prioritizing the distribution of oral antivirals to those who do not require supplemental oxygen but at the highest risk of disease progression. Our study cohort reflected such a prescription pattern in real-world clinical practice, consisting of mostly older people with multiple pre-existing comorbidities and who had not been fully vaccinated," said Dr. Carlos Wong King-ho, Assistant Professor of the Department of Pharmacology and Pharmacy, HKUMed.
"The antiviral effect and mortality benefit observed in this patient cohort support the use of oral antivirals in patients with COVID-19 who do not require supplemental oxygen on admission during a pandemic wave of the omicron variant."
In conclusion, this retrospective cohort study of hospitalized patients with COVID-19 who did not initially require supplemental oxygen showed that early initiation of oral antivirals was associated with significant reductions in risk of all-cause mortality and disease progression, and with reaching a low viral burden faster than non-use, during an epidemic dominated by the SARS-CoV-2 omicron BA.2 subvariant.
These findings support the use of these antivirals in this population. As both oral antivirals are currently indicated for non-hospitalized patients with COVID-19 who are at high risk of disease progression, ongoing research will inform the safety and effectiveness of oral antivirals in specific patient populations, drug combinations, and health care settings.
More information: Carlos K H Wong et al, Real-world effectiveness of early molnupiravir or nirmatrelvir–ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, The Lancet Infectious Diseases (2022). DOI: 10.1016/S1473-3099(22)00507-2