The gut microbiome's supersized role in shaping molecules in our blood
Scientists from the Institute for Systems Biology (ISB) have shown which blood metabolites are associated with the gut microbiome, genetics, or the interplay between both. Their findings will be published in the journal Nature Metabolism and have promising implications for guiding targeted therapies designed to alter the composition of the blood metabolome to improve human health.
The nearly 200-year-old phrase "you are what you eat" has some new evidence. ISB researchers have found that the gut microbiome, including what we feed it, is largely responsible for the variation in circulating blood metabolites across people. This knowledge will help guide targeted interventions designed to alter the composition of the human blood metabolome. The findings will be published in Nature Metabolism on Thursday, November 10.
"We know that person-to-person variation in the blood metabolome—the small molecules found in the bloodstream that can interact with all the systems of our body—can tell us a lot about health and disease status. Figuring out what governs this variation is a necessary step that gets us closer to precision approaches to health care," said Dr. Sean Gibbons, an ISB faculty member and co-corresponding author of the paper.
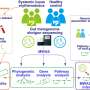
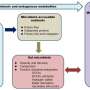
The research team examined 930 blood metabolites that were present in more than 1,500 individuals. More than 60% of the detected metabolites were significantly associated with either host genetics or the gut microbiome. "Notably, 69% of these associations were driven solely by the microbiome, with 15% driven solely by genetics and 16% were under hybrid genetic-microbiome control," said ISB Senior Research Scientist Dr. Christian Diener, lead author of the study. Diener and co-lead author Chengzhen Dai analyzed the de-identified metabolomic, genomic, and microbiome data from consenting patients in a consumer scientific wellness program.
They found that the blood metabolite variation explained by the microbiome was largely independent of the variation explained by the genome, even for hybrid metabolites that were significantly associated with both genetics and microbes. Additionally, certain metabolite-microbe associations were only significant in individuals with specific genetic backgrounds, indicating a nuanced interplay between the microbiome and host genetics in shaping the blood metabolome.
These new findings are promising for a couple of reasons. First, the high number of microbiome-specific metabolites suggests that much of our blood metabolome could be modified through dietary, probiotic, and other lifestyle interventions. Second, metabolites that are under stricter genetic control may not be responsive to lifestyle modification, making them targets for pharmacological interventions that directly target host pathways.
"A deeper understanding of the determinants of the blood metabolome will provide us with a window into how these circulating metabolite levels can be engineered and optimized for health," said Dr. Andrew Magis, co-corresponding author of the paper. "Understanding which circulating small molecules fall predominantly under host versus microbiome control will help guide interventions designed to prevent and/or treat a range of diseases."
More information: Andrew Magis, Genome–microbiome interplay provides insight into the determinants of the human blood metabolome, Nature Metabolism (2022). DOI: 10.1038/s42255-022-00670-1. www.nature.com/articles/s42255-022-00670-1