New recommendations for reporting in synovial tissue research in rheumatology
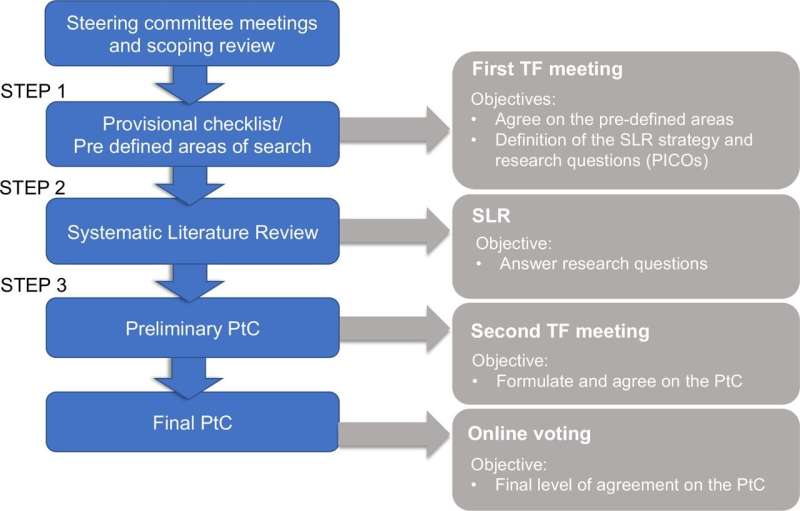
The new European Alliance of Associations for Rheumatology (EULAR) points-to-consider were developed by a multidisciplinary task force of 25 members from 10 European countries. The people taking part had expertise in rheumatology, immunology, and pathology. The group also included allied health professionals and patient representatives. The work was completed in line with EULAR standardized operating procedures and based on a systematic literature review that was conducted to gather evidence.
Analysis of synovial tissue may offer a promising approach for personalized therapy in rheumatic diseases. This technology could also advance our understanding of disease mechanisms and help identify new therapeutic targets. However, although there is a great deal of research in synovial tissue, there are inconsistencies in the way the tissue is handled, as well as about how information collected is reported.
The new paper developed by EULAR and published in February 2022 issue of the Annals of the Rheumatic Diseases includes three overarching principles and nine points to consider.
The principles say that synovial biopsies are safe and well-tolerated when performed in aseptic conditions, and can be performed for both clinical and research purposes—but should be guided by imaging techniques. Arthroscopy and ultrasound are the preferred techniques to guide synovial biopsies, and can be used without affecting the tolerability of the procedure for the patient, or reducing the amount of tissue required for meaningful analysis.
The nine points to consider cover how details of the biopsy procedure should be reported, as well as aspects of study design and tissue handling and processing methods. From a clinical point of view, they also suggest that conventional patient disease activity measures, disease stage, and treatment should be described to allow evaluation of the generalizability and validity of the outcome.
EULAR hope these points to consider will serve as a reference and checklist for clinicians and scientists involved in publishing, reviewing, and reading manuscripts reporting on research in synovial tissue.
More information: Aurélie Najm et al, EULAR points to consider for minimal reporting requirements in synovial tissue research in rheumatology, Annals of the Rheumatic Diseases (2022). DOI: 10.1136/annrheumdis-2021-221875