This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Type 1 diabetes: First clinical trial of GABA/GAD in newly diagnosed, very young children
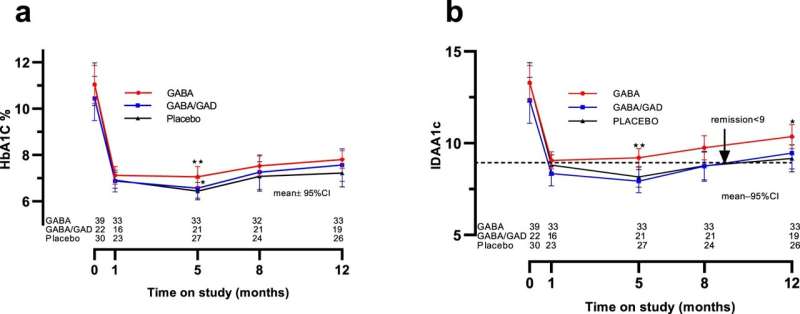
For the first time, humans with newly diagnosed type 1 diabetes, or T1D, have received two treatments called GABA and GAD that have shown promise in animal studies and in isolated human pancreas islets. This investigator-initiated clinical trial, published in Nature Communications, focused exclusively on children with recent onset T1D.
Diabetes is a disease affecting two pancreatic hormones—insulin and glucagon. In healthy people, insulin helps cells take up glucose from the blood when glucose levels are high. In contrast, glucagon helps the liver release glucose into the bloodstream when glucose levels are low. Thus, levels of blood glucose remain steady.
In T1D, autoantibodies destroy the pancreatic beta cells, insulin release is diminished, and glucagon release is excessive relative to the insulin deficiency. This can cause a vicious cycle of escalating blood glucose levels. Strategies to ameliorate or cure T1D, therefore, target the preservation of insulin-secreting beta cells and/or attenuation of the relative excess of alpha cell glucagon. Most importantly, concerning the inhibition of alpha cell glucagon in this trial by GABA/GAD, recent studies in animals made diabetic have shown that inhibition of glucagon leads to expansion of insulin-secreting beta cells and improvements in hyperglycemia.
Researchers in the study, led by University of Alabama at Birmingham physicians, were able to enroll children within the first five weeks of diagnosis, before the near total eradication of beta cells. Forty percent of the study participants were younger than 10 years old. The study—which was constrained to lower-dose GABA therapy by the United States Food and Drug Administration because it was the first human trial with GABA—did not achieve its primary outcome, the preservation of insulin production by beta cells.
However, it did meet the clinically relevant secondary outcome of reduced serum glucagon. Significantly, the trial confirmed the safety and tolerability of oral GABA. Additionally, in collaboration with the immunology team of Hubert Tse, Ph.D., at the UAB Comprehensive Diabetes Center, a separate manuscript under review will describe a salutary effect of GABA alone and in combination with GAD on cytokine responses in peripheral blood mononuclear cells from trial participants.
GABA is gamma aminobutyric acid, a major inhibitory neurotransmitter. In the endocrine pancreas, GABA participates in paracrine regulation—meaning a hormone that acts on nearby cells—on the beta cells that produce insulin and the alpha cells that produce glucagon.
In various mouse model studies, GABA was able to delay diabetes onset, and restore normal blood glucose levels after diabetes had already commenced. GABA treatment also led to significant decreases in the inflammatory cytokine expression that participates in the pathogenesis of T1D.
GAD is glutamic acid decarboxylase, the enzyme that acts on glutamate to form GABA. Animal and pancreatic islet cell studies show that immunization with GAD alone may help preserve beta cells. Both GABA and GAD are highly concentrated in the pancreatic islet, which is the autoimmune target of T1D.
The study, which was conducted between March 2015 and June 2019, screened 350 patients and enrolled 97, whose ages averaged 11 years. Forty-one took oral GABA twice a day; 25 took the oral GABA in combination with two injections of GAD, one at the baseline visit and one at the one-month visit. The remaining 31 children received a placebo treatment. Analysis after one year of treatment included 39 in the GABA group, 22 in the GABA/GAD group and 30 in the placebo group.
"Given that GABA reduces immune inflammation at higher doses in several diabetic rodent models, it is plausible that increased GABA doses, or longer-acting preparations, could offer sufficiently prolonged, above-threshold GABA concentrations to preserve islet cells, particularly during stage 1 diabetes," said Gail Mick, M.D., a UAB professor in the Department of Pediatrics' Division of Pediatric Endocrinology and Diabetes. Mick and Kenneth McCormick, M.D., who recently retired from UAB Pediatrics, co-led the trial.
More information: Alexandra Martin et al, A randomized trial of oral gamma aminobutyric acid (GABA) or the combination of GABA with glutamic acid decarboxylase (GAD) on pancreatic islet endocrine function in children with newly diagnosed type 1 diabetes, Nature Communications (2022). DOI: 10.1038/s41467-022-35544-3