This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Differences in animal biology can affect cancer drug development
A small but significant metabolic difference between human and mouse lung tumor cells, has been discovered by Weill Cornell Medicine researchers, explaining a discrepancy in previous study results, and pointing toward new strategies for developing cancer treatments.
The work, published in Cancer Discovery, focuses on lung adenocarcinoma, a common but often difficult to treat cancer that researchers have long studied in mouse models. However, those models didn't quite align with human clinical observations in some instances. The new paper shows why; a specific gene mutation has opposite effects on tumor growth in mice and humans.
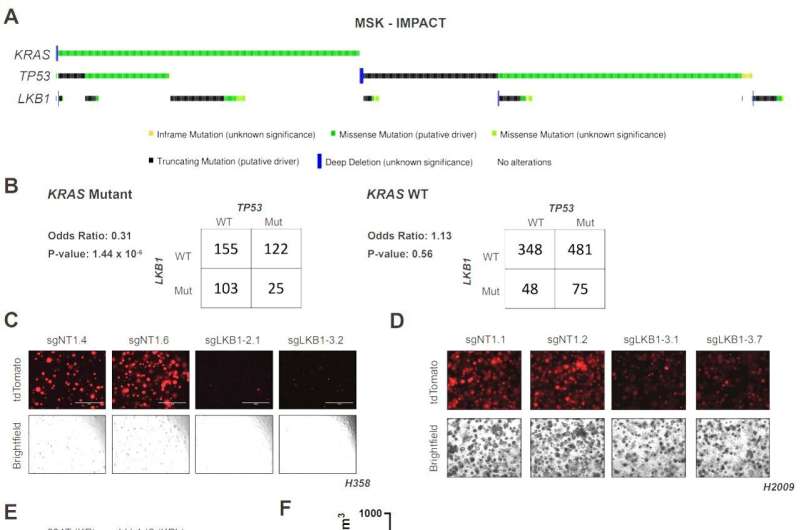
The inspiration for the project dates back more than a decade, when co-lead author Dr. Benjamin Stein, now an instructor of cancer biology in medicine at Weill Cornell Medicine, was studying the role of the tumor suppressor LKB1 in metabolism as a graduate student. In mice, tumors containing mutations in two key genes, KRAS and TP53, can become much more aggressive by acquiring a mutation in the third gene, LKB1.
"But the clinical data showed that the three mutations didn't really co-occur with high frequency in humans," said Dr. Stein. Upon starting his post-doctoral appointment in the laboratory of co-senior author Dr. Lewis Cantley, the former director of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine, Dr. Stein decided to focus on decoding this difference.
At the time, co-lead author Dr. John Ferrarone was pondering the same observation in the human clinical data next door, as a medical oncology fellow working in the laboratory of co-senior author Dr. Harold Varmus, the Lewis Thomas University Professor of Medicine and a member of the Meyer Cancer Center.
"My main interest in this project was more from a clinical angle, so I was interested in learning if there was a way to potentially exploit tumors with these common mutations," said Dr. Ferrarone, who is now an instructor in medicine at Weill Cornell.
Teaming up with Dr. Varmus, Dr. Cantley, who is now at the Dana Farber Cancer Center, and additional collaborators across Weill Cornell and at other institutions, the researchers embarked on a deep dive into the way the products of the three genes behave in mouse models and cultured human cells. The group employed techniques that ranged from the cutting edge of genetic engineering, proteomics, metabolomics and mouse models to classical biochemical and cell biological experiments.
"Using multiple approaches at the genetic, protein and metabolite level allowed us to obtain a more comprehensive understanding of what was going on within each species, and then try to cross-correlate and dissect where the disparities were between the two," said Dr. Stein.
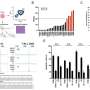
The multi-disciplinary approach pinpointed a crucial difference in regulation of glucose metabolism between mice and humans driven by LKB1, due to slight differences in the two species' regulation of the metabolic enzyme triose phosphate isomerase (TPI1).
As a result, mouse tumors that carry mutations in KRAS and TP53 gain a significant metabolic advantage by mutating LKB1 as well. But, in humans, mutating LKB1 in the same context appears to harm the tumor cells as they can no longer regulate TPI1 and glucose metabolism. That explains why the three mutations seldom occur together in human cancers.
Those results suggest a new strategy for attacking lung adenocarcinomas: targeting the metabolic pathway LKB1 controls. "Taking out a master regulator enzyme can have very detrimental effects, so the discussions now are more about going downstream and targeting other molecules," said Dr. Stein.
Nonetheless, he and Dr. Ferrarone are optimistic about the work guiding future drug discovery efforts. The new findings also sound a cautionary note for investigators relying heavily on mouse models to screen drug candidates, as compounds that might work well against human cancers could actually be missed in current murine tumor models.
More information: Benjamin D. Stein et al, LKB1-dependent regulation of TPI1 creates a divergent metabolic liability between human and mouse lung adenocarcinoma, Cancer Discovery (2023). DOI: 10.1158/2159-8290.CD-22-0805