This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Early-life stress can disrupt maturation of brain's reward circuits, promoting disorders
A new brain connection discovered by University of California, Irvine researchers can explain how early-life stress and adversity trigger disrupted operation of the brain's reward circuit, offering a new therapeutic target for treating mental illness. Impaired function of this circuit is thought to underlie several major disorders, such as depression, substance abuse and excessive risk-taking.
In an article recently published online in Nature Communications, Dr. Tallie Z. Baram, senior author and UCI Donald Bren Professor and Distinguished Professor in the Departments of Anatomy & Neurobiology, Pediatrics, Neurology and Physiology & Biophysics, and Matt Birnie, lead author and a postdoctoral researcher, describe the cellular changes in the brain's circuitry caused by exposure to adversity during childhood.
"We know that early-life stress impacts the brain, but until now, we didn't know how," Baram said. "Our team focused on identifying potentially stress-sensitive brain pathways. We discovered a new pathway within the reward circuit that expresses a molecule called corticotropin-releasing hormone that controls our responses to stress. We found that adverse experiences cause this brain pathway to be overactive."
"These changes to the pathway disrupt reward behaviors, reducing pleasure and motivation for fun, food and sex cues in mice," she said. "In humans, such behavioral changes, called 'anhedonia,' are associated with emotional disorders. Importantly, we discovered that when we silence this pathway using modern technology, we restore the brain's normal reward behaviors."
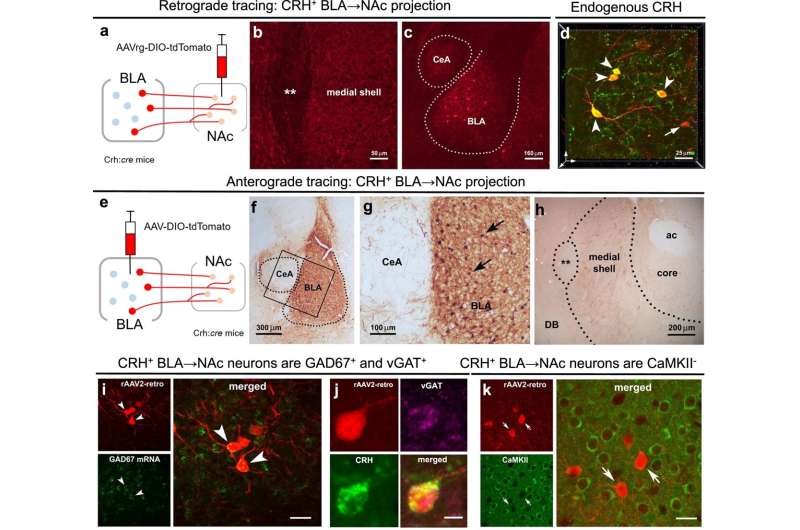
Researchers mapped all the CRH-expressing connections to the nucleus accumbens, a pleasure and motivation hub in the brain, and found a previously unknown projection arising from the basolateral amygdala. In addition to CRH, projection fibers co-expressed gama-aminobutyric acid. They found that this new pathway, when stimulated, suppresses several types of reward behaviors in male mice.
The study involved two groups of male and female mice. One was exposed to adversity early in life by living for a week in cages with limited bedding and nesting material, and the other was reared in typical cages. As adults, the early adversity-experiencing male mice had little interest in sweet foods or sex cues compared to typically reared mice. In contrast, adversity-experiencing females craved rich, sweet food. Inhibiting the pathway restored normal reward behaviors in males, yet it had no effect in females.
"We believe that our findings provide breakthrough insights into the impact of early-life adversity on brain development and specifically on control of reward behaviors that underlie many emotional disorders. Our discovery of the previously unknown circuit function of the basolateral amygdala-nucleus accumbens brain pathway deepens our understanding of this complex mechanism and identifies a significant new therapeutic target," Baram said.
"Future studies are needed to increase our understanding of the different and sex-specific effects of early-life adversity on behavior."
More information: Matthew T. Birnie et al, Stress-induced plasticity of a CRH/GABA projection disrupts reward behaviors in mice, Nature Communications (2023). DOI: 10.1038/s41467-023-36780-x