This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New drug to lower brain pressure could treat blinding IIH headaches, trial finds
Patients with "blinding" headaches known as Idiopathic Intercranial Hypertension (IIH) could be treated with an injectable peptide used for type 2 diabetes, a new trial has found.
The study, published in the journal Brain, today reports on a phase two trial of a drug called exenatide, a GLP-1 receptor agonist, as a potential treatment for IIH.
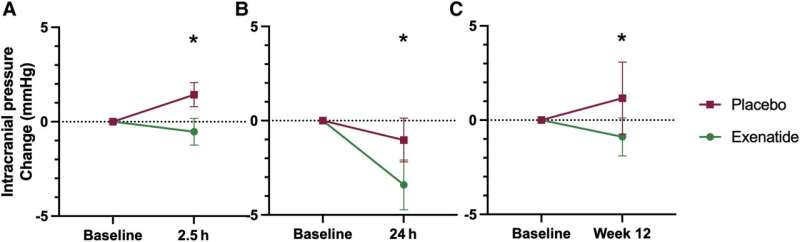
The IIH Pressure Trial led by a team of neurologists from the University of Birmingham and University Hospitals Birmingham found that for the seven patients who received regular injections of the drug, currently approved for use in Type 2 Diabetes, led to a drop in pressure in the brain during both short (2.5hrs and 24hrs) and long term (12 weeks) measurements.
The trial also saw significant reductions in the numbers of headaches across the 12 weeks that participants took part, with an average of 7.7 fewer days per month of headaches compared to the baseline, compared to only 1.5 fewer days in the placebo arm.
Alex Sinclair is Professor of Neurology in the Institute of Metabolism and Systems Research at the University of Birmingham, an Honorary Consultant Neurologist at University Hospitals Birmingham NHS Foundation Trust, and Principal Investigator of the study. Professor Alex Sinclair said, "This is a major trial for the rare and debilitating condition IIH that can lead to people, usually women, going blind and suffering disabling daily headaches. There are no current licensed drugs to treat IIH and hence this result is a major step forward for IIH patients.
"We are delighted to see that the phase two trial resulted in our treatment group having lower brain pressure both immediately and after 12 weeks and nearly eight fewer headache days across the 12-week period, and that all the women were able to continue the treatment throughout with few adverse effects. We now hope to see a much larger trial of exenatide to literally ease the pressure for the many people around the world suffering with IIH."
Shot in the arm for IIH treatment
Idiopathic Intracranial Hypertension (IIH) is a debilitating condition that raises pressure in the brain and can lead to chronic headaches and even permanent sight loss. The illness, which often leaves patients with a reduced quality of life, predominately affects women aged 25 to 36 and weight gain is a major risk factor of developing IIH and relapses of the disease.
Once regarded as rare, incidence of IIH is now rising dramatically in line with the global rise in obesity and there has been a 350% rise in incidence in last 10 years. Currently there are no licensed drugs options and existing medications used off label are complicated by troublesome side effects.
A key finding was the rapid action of the drug, with results indicating that brain pressure was significantly reduced within two and half hours of taking the medication. This rapid onset of action is vital in a condition which can cause rapid blindness if left untreated.
Dr. James Mitchell, Lecturer in Neurology at the University of Birmingham and first author of the paper said, "The results of this clinical trial are a shot in the arm for finding clinical treatments for IIH. While we need to do further trials before such a treatment could be available for patients in the future, we are encouraged by the significant results from this trial that made a real difference for those in the treatment arm and this treatment may prove relevant for other conditions resulting in raised brain pressure."
In this study the drug was given as a twice daily injection into the subcutaneous tissue. To reduce the need for frequent injection in the future a once weekly subcutaneous injection called Presendin will be trialed though University of Birmingham Start-up company, Invex Therapeutics.
Shelly Williamson, the Chair of patient charity IIH UK said, "This is such exciting progress. New drug options is vitally important for IIH and this trial brings hope to the millions of patients living with the condition. We very much look forward to the next steps and seeing the drug tested in two large Phase 3 clinical trials."
The IIH Advance is a Phase 3 clinical trial in Adolescents run in the U.K., sponsored by the University of Birmingham and IIH Evolve is running in adults internationally sponsored by Invex Therapeutics. Ultimately the aim is to gain enough evidence to allow the drug to be licensed for use in IIH patients in the future.
More information: James Mitchell et al, The effect of GLP-1RA exenatide on Idiopathic Intracranial Hypertension: Randomised Clinical Trial, Brain (2023). DOI: 10.1093/brain/awad003