This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Genetic test could guide use of cancer chemotherapy
Researchers from Imperial College London, the Institute of Cancer Research (ICR) and the Netherlands Cancer Institute, found that the test can predict whether a bowel cancer patient will benefit from chemotherapy. It is thought that this could spare patients who will not benefit from treatment from unnecessary toxicity and debilitating side effects.
The genetic test is already used as part of standard of care in the UK and worldwide to predict patients' responses to other targeted cancer drugs, meaning doctors could apply it to guide chemotherapy straight away. In bowel cancer, responses to last-line chemotherapy treatment trifluridine/tipiracil vary greatly between patients—with some patients showing good, long-term responses, and others seeing no benefits.
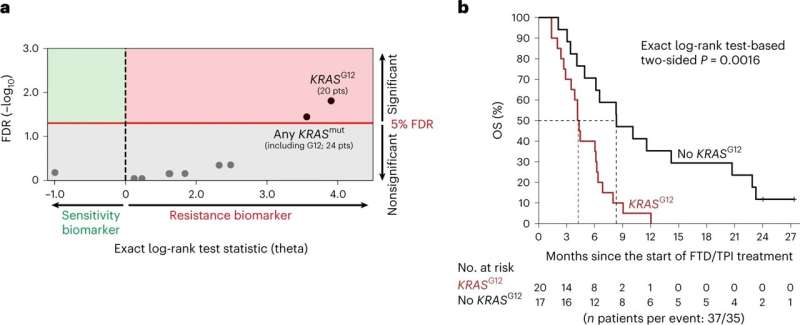
The researchers found that a specific mutation in the KRAS gene called KRASG12 was linked to poor survival in treated patients. Conversely another mutation was linked to a three-fold increase in survival.
Professor Nicola Valeri, Honorary Professor of Gastrointestinal Oncology at Imperial College London and the ICR, said, "This is the first time we have a genomic marker already used in the clinic that can tell us whether a patient's cancer will be sensitive or resistant to chemotherapy. We hope doctors will use this data to improve care for patients with advanced bowel cancer without delay."
"It will be difficult for some patients to find out that this last-line drug will not benefit them, but this test will mean they are able to avoid unnecessary side effects and have a better quality of life with advanced cancer. Fortunately, our findings also reveal a group of patients who see substantial benefits from taking this type of chemotherapy."
The researchers are calling for regulators to rapidly incorporate the findings into guidelines so that using the test to direct treatment with trifluridine/tipiracil becomes standard of care.
Professor Kristian Helin, Chief Executive of The Institute of Cancer Research, London, said, "Treating cancer well is not just about allowing people to live for longer, but also about giving them the best possible quality of life. Although chemotherapy can be very effective for many patients, it can also have debilitating side effects—and so it is important to have as much information as possible about how likely treatment is to work."
"This is an exciting advance for genomic medicine—showing that genomic tests are not just useful for guiding treatment with targeted therapies, but also harsher chemotherapies. It will have a big impact for people with advanced bowel cancer, by taking the guesswork out of their treatment."
The paper is published in the journal Nature Medicine.
More information: Joris van de Haar et al, Codon-specific KRAS mutations predict survival benefit of trifluridine/tipiracil in metastatic colorectal cancer, Nature Medicine (2023). DOI: 10.1038/s41591-023-02240-8