This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers discover two subtypes of insulin-producing cells
A team led by Van Andel Institute and Max Planck Institute of Immunobiology and Epigenetics scientists has identified two distinct subtypes of insulin-producing beta cells, or ß cells, each with crucial characteristics that may be leveraged to better understand and treat type 1 and type 2 diabetes.
ß cells are critical guardians of the body's metabolic balance. They are the only cells capable of producing insulin, which regulates blood sugar levels by designating dietary sugar for immediate use or storage.
In type 1 diabetes, ß cells are attacked by the body's own immune system, rendering them unable to produce insulin.
Type 2 diabetes arises from insulin resistance; the resulting excess blood sugar from a person's diet causes ß cells in the pancreas to work overtime. Eventually, ß cells can no longer keep up and blood sugar concentrations can rise to dangerously high levels.
Both diseases are treated by enhancing insulin action, either by providing insulin itself, or by augmenting its activity and release into the blood. Some people with type 1 diabetes may elect to have a ß cell transplant, an experimental procedure in which functioning cells from a donor are implanted into the pancreas.
The new findings, published in Cell Metabolism, suggest several potential paths that could inform future diabetes treatments, such as adjusting the ratio of ß cell subtypes in transplants to ensure optimal function.
"All cells vary in some way, but these two ß cell subtypes are discretely and consistently different from one another. This indicates that they serve two different but necessary functions as insulin producers. They are specialists, each with their own roles," said J. Andrew Pospisilik, Ph.D., a Van Andel Institute professor and senior author of the study. "We also see differences in the ratio of one subtype to another in diabetes. Understanding these two cell types—and their relationship to each other—gives us a clearer picture of diabetes and offers new opportunities for treatment."
Scientists have long recognized differences among ß cells, but this study is the first to clearly delineate specific cell subtypes. The findings were identified in both mouse models and in human ß cell samples.
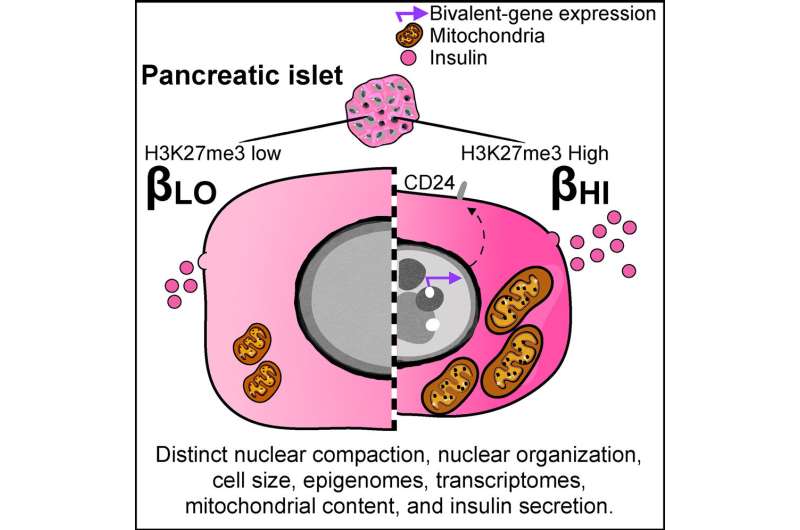
The two types—described by study authors as ßHI and ßLO—differ in specific function, size, shape, and epigenomic features, among other characteristics. They also exhibit contrasting patterns of surface markers, which help cells send and receive chemical messages. ßHI cells appear to be more prevalent in type 2 diabetes.
Importantly, the subtypes can be separated by the presence or absence of a protein called CD24, which acts as a marker that allows targeting of one type and not the other. This distinction may inform development of more precise diabetes treatment strategies and offers a critical tool that enables diabetes researchers to better study each cell type in depth.
The findings also reshape what is known about how ß cells develop early in life. ß cells are among the longest-lived cells in the body, with lifespans of 30 to 40 years. Like all cells, the earliest ß cells arise from stem cells, which are blank slates that differentiate into the many cell types that comprise the body. This process is largely guided by specialized proteins called transcription factors, which switch genes "on" and "off."
However, the study suggests ß cells may be an exception. The researchers identified epigenetic dosage rather than transcription factors as a driving force behind the decision of ß cells to be become ßHI or ßLO. This is the first time epigenetic dosage has been shown to change the ratio of related cell types.
Like transcription factors, epigenetic marks tell genes when to be active and when to be silent. Epigenetic dosage refers to the amount of these marks. In ß cells, the team previously identified an epigenetic mark called H3K27me3 as a key driver of differentiation. In this new study, they found that dosage of the same mark controls ßHI versus ßLO numbers and, as a result, offers a new target for potential new diabetes treatments.
"The beauty of this mechanism is its novelty—it's purely driven by epigenetics with no help from transcription factors," Pospisilik said. "The key here is that epigenetic changes can be reversed, which opens a whole host of questions with implications for treatment."
More information: Erez Dror et al, Epigenetic dosage identifies two major and functionally distinct β cell subtypes, Cell Metabolism (2023). DOI: 10.1016/j.cmet.2023.03.008