This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How cancer cells muscle their way into other organs
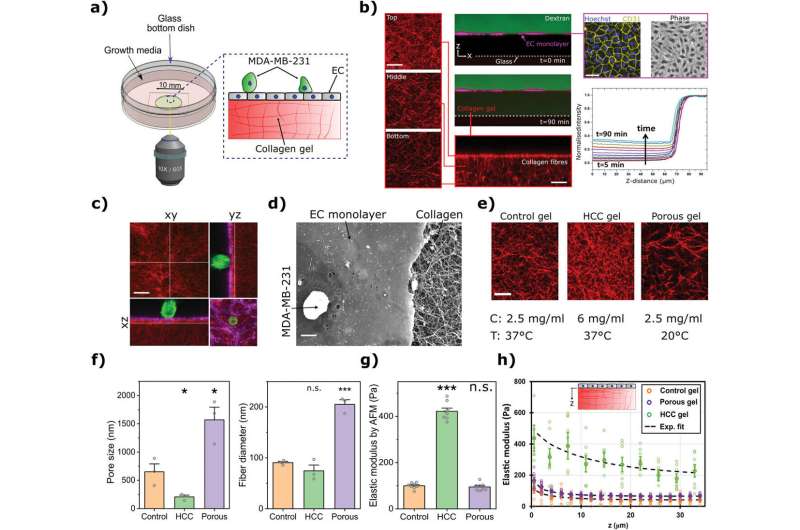
The biomechanics of how cancer cells escape from the bloodstream to invade other organs has been described for the first time by researchers from UCL, MIT and their collaborators.
The study, published in Advanced Science, found that the more porous and the softer the tissue, the more likely cancer cells were to force their way in and were able to do so more quickly, providing valuable data for research seeking to prevent or halt cancer metastasis, which is the leading cause of cancer mortality.
Drugs that influence the stiffness of the environment around tumors—such as PAT-1251 and PXS-5505—are being tested in clinical trials for cancers that have not yet spread, and the hope is that similar approaches could be used to treat metastasized cancers.
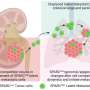
Solid cancers emerge in one place in the body, known as the primary tumor site. Metastasis is when cancer cells break away from the primary tumor and spread to other parts of the body via the bloodstream or lymphatic system, which can lead to secondary tumors in other organs. The process of cancer cells breaking out of the circulatory system into other tissues is known as extravasation.
Most cancer research focuses on spotting cancers early and treating them before they spread, which has resulted in improved outcomes for patients. But once a cancer has metastasized it becomes much harder to treat, underlined by the fact that metastasis is a factor in the vast majority of cancer deaths. Though metastasized cancers can be treated, they cannot be cured.
In this study, researchers from UCL and MIT set out to better understand the biomechanical forces that determine how cancer cells are able to invade other tissues from the bloodstream.
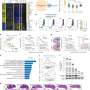
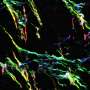
They created in vitro models of the endothelium, a single-cell layer that separates the bloodstream from other tissues, and placed it over different preparations of collagen gel to mimic stiffer or more porous tissues. Tumor cells were then introduced to the models to observe whether they could penetrate the endothelium, how long this took and how they managed to do so in mechanical terms.
Dr. Yousef Javanmardi (UCL Mechanical Engineering), first author of the study, said, "We observed that cancer cells need to latch onto the endothelium to establish a hold, which they use to push and pull themselves through this tightly-woven cell layer and into the tissue beyond. It's like if you were stuck in thick mud: you'd need to use something solid like a rock to be able to pull and push yourself to firmer ground. The thicker the mud, the harder that would be. You might not get out and if you did, it'd take longer."
The findings raise the question of whether the biomechanical properties of specific tissues in the body determine where secondary tumors form.
Given the extreme difficulty in studying metastasis in this manner in human tissues, the next step will be to create more advanced three-dimensional models of the vascular system to observe cancer biomechanics in more detail in conditions that better imitate human biology. This would allow drugs to be tested and would hopefully identify candidates for human trials.
Professor Emad Moeendarbary (UCL Mechanical Engineering), a senior author of the study, said, "It is hugely satisfying to see this highly interdisciplinary and collaborative research come to fruition after five years. The challenge of studying metastasis is significant and it has taken a huge effort to produce these findings, involving physicists, biologists, engineers, mathematicians and oncologists. It is a great advert for the power of collaboration and I am optimistic that these data will help further our understanding of cancer metastasis and the development of drugs to treat it."
More information: Yousef Javanmardi et al, Endothelium and Subendothelial Matrix Mechanics Modulate Cancer Cell Transendothelial Migration, Advanced Science (2023). DOI: 10.1002/advs.202206554