This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers discover critical protein for common bone growth disorder
A team led by UT Southwestern Medical Center researchers has discovered a protein that appears to be pivotal for traumatic heterotopic ossification (HO), a condition in which bone forms in muscles, tendons, and other soft tissues after traumatic injury or surgery. The findings, published in Science Advances, could yield new ways to prevent this common complication, the researchers say.
"Right now, we have no ways to prevent HO from occurring, which can cause significant impairments in quality of life. For example, it can alter patients' range of motion, producing chronic pain, and affect the ability of amputees to fit into prostheses," said Benjamin Levi, M.D., Associate Professor of Surgery and Plastic Surgery, and Chief of the Division of Burn, Trauma, Acute, and Critical Care Surgery at UT Southwestern. "Hopefully, these new findings will lead to treatments that can stop this process from unfolding so patients never develop it."
HO occurs when cells that serve as precursors to various tissues in the musculoskeletal system abnormally develop into bone and/or cartilage following surgery, such as joint replacement, or traumatic injuries, such as blast injuries of combat veterans, head injuries, burns, and joint dislocations. As many as 50% of patients who undergo total hip replacements develop HO, said Chase A. Pagani, a UT Southwestern M.D./Ph.D. student in the Levi lab who co-led the study with Dr. Levi.
Despite the high frequency of HO, Mr. Pagani explained, little is known about how or why it develops. After observing that it frequently occurs around joints, members of the Levi lab conducted earlier research showing that immobilizing joints in animal models with this condition significantly reduced bone formation by preventing collagen fibers surrounding the injury from aligning. Although these findings suggested HO occurs from an interaction between the precursor cells and collagen, the underlying molecular mechanism remained a mystery.
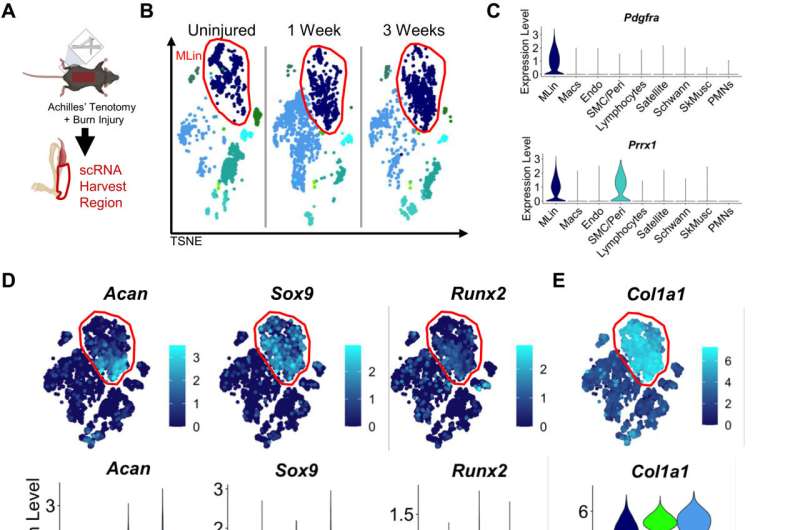
To better understand HO, Dr. Levi, Mr. Pagani, and their colleagues collected cells from the Achilles tendons of lab mice before induction of HO, then one and three weeks after induction. When they examined the cells' gene activity, they homed in on a gene called Ddr2, which produces a protein known as discoidin domain receptor 2 (DDR2). This protein, which lies on the surfaces of cells, binds collagen and has been linked to bone and cartilage formation.
When the researchers deleted DDR2 by genetic techniques or inactivated it by treating the mice with a drug, HO formation lessened significantly. A closer look at the collagen surrounding the HO showed that removing this protein prevented collagen fibers from aligning, much like immobilizing joints had in the previous study.
These results show that DDR2 is a crucial player for traumatic HO, Dr. Levi said. The drug used to suppress this protein in the study is FDA-approved to treat cancer, and other drugs in development could offer more specific DDR2 targeting—suggesting that an effective HO prevention and/or treatment may already exist.
More information: Chase A. Pagani et al, Discoidin domain receptor 2 regulates aberrant mesenchymal lineage cell fate and matrix organization, Science Advances (2022). DOI: 10.1126/sciadv.abq6152