This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
EU watchdog backs GSK's RSV vaccine for over 60s
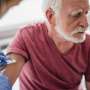
The European Union's drug watchdog has recommended approval of pharmaceutical giant GSK's vaccine against the RSV respiratory virus for over-60s, the British firm said on Thursday.
There are currently no authorised shots for the common virus, which causes cold-like symptoms in most cases but can be serious or even deadly in vulnerable older people.
However, pharma firms including GSK, Pfizer and Moderna have all developed vaccines against the respiratory syncytial virus (RSV) that they are racing to get authorised.
If approved, GSK's shot has "the potential to be the first vaccine available to help protect older adults from RSV disease," the company said in a statement.
The European Medicines Agency said its committee for human medicines gave a positive assessment of the vaccine based on the results of a trial which included 25,000 participants in 17 countries.
The results showed that the vaccine was 83 percent effective at protecting against RSV-related diseases in people aged 60 years or over, with generally mild side effects.
The positive opinion will now be evaluated by the European Commission, which normally follows the EMA's recommendations to approve drugs.
GSK said that a decision was expected by July.
The vaccine uses an engineered protein and an adjuvant substance to promote antibodies and T cells that help fight off RSV infection.
RSV causes 250,000 people over 60 to be hospitalised and 17,000 to die every year in Europe, according to the EMA.
GSK said its vaccine is also being reviewed in the United States, Japan and other countries.
Pfizer has said that it expects a decision from the US Food and Drug Administration in May for its own over-60s RSV vaccine.
In January, Moderna said it hopes its RSV vaccine would be approved and available for the Northern Hemisphere's winter later this year.
Last year, the EU approved a preventative treatment against RSV, developed British-Swedish pharmaceutical firm AstraZeneca and France's Sanofi, which works similarly to a vaccine.
© 2023 AFP