This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
International panel classifies immunotherapy side effects for the first time
An international panel of oncology and immunotherapy experts led by RCSI's Professor Jarushka Naidoo, has developed standardized definitions of the side effects of cancer immunotherapy to assist clinicians in treating patients.
Immunotherapies have revolutionized the treatment of solid tumor cancers, improving outcomes for patients globally. However, the anti-tumor mechanisms of these therapies are known to cause side effects called immune-related adverse events (irAEs).
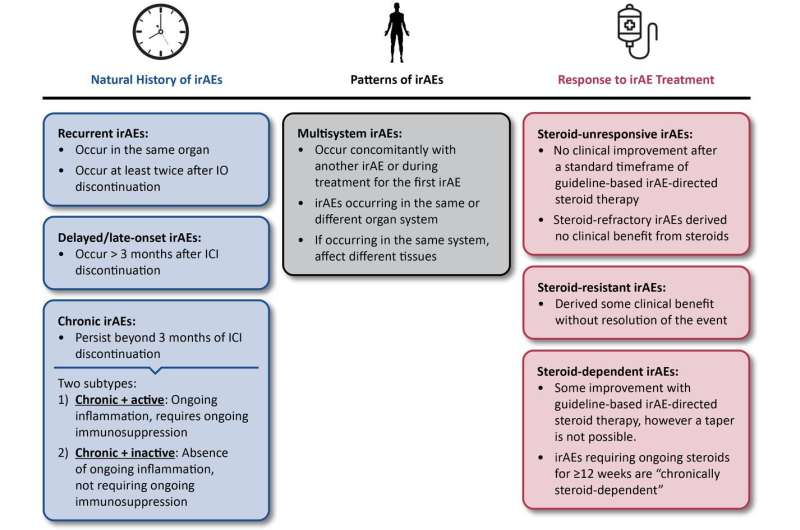
The paper, published in the Journal for Immunotherapy of Cancer is the first set of consensus expert definitions relating to the diagnosis and management of side effects caused by immunotherapy, specifically immune checkpoint inhibitors. They provide a shared vocabulary for clinicians which will help standardize the application of clinical practice guidelines and support clinicians in offering the best treatment to patients.
Commenting on the publication, first author Professor Jarushka Naidoo, Academic Professor of Medical Oncology in the RCSI Department of Medicine, said, "This set of guidelines will prove an important resource in cancer immunotherapy clinical trials as well as enabling more research into the biomarkers that help us predict adverse effects in patients."
Professor Naidoo and co-author Dr. Catherine Murphy, Oncology Specialist Registrar, Beaumont RCSI Cancer Centre assembled an expert panel with the Society for Immunotherapy of Cancer (SITC) to develop the definitions, addressing the unmet need for uniform terminology for different types of immune-related adverse events.
These had previously been inconsistently documented in literature and vary widely from short term inflammation to damage to organs. Over twenty oncology and immunotherapy specialists from academic medicine, industry, patient advocacy and regulatory agencies were involved.
Immunotherapy helps the body to find and destroy cancer cells. It can treat many different types of cancer and can be used alone or in parallel with other cancer treatments such as chemotherapy.
More information: Jarushka Naidoo et al, Society for Immunotherapy of Cancer (SITC) consensus definitions for immune checkpoint inhibitor-associated immune-related adverse events (irAEs) terminology, Journal for ImmunoTherapy of Cancer (2023). DOI: 10.1136/jitc-2022-006398