This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New study defines analytical performance specifications for 24,25-dihydroxyvitamin D examinations
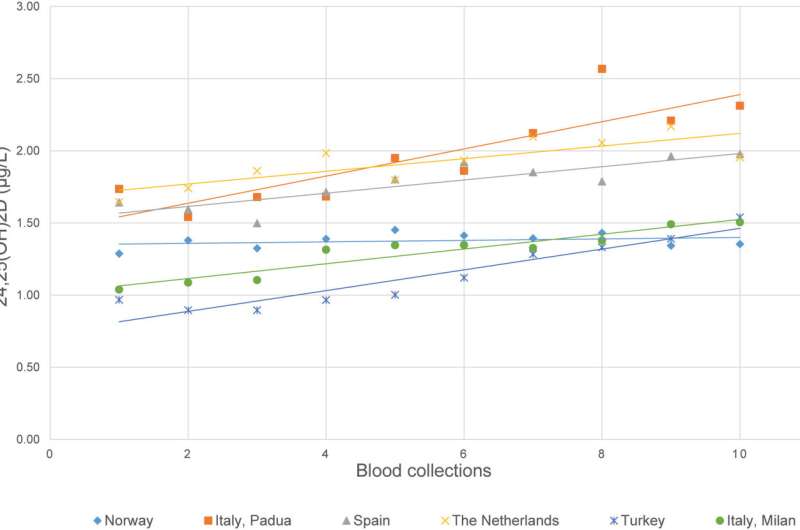
The first study to define analytical performance specifications for 24,25-dihydroxyvitamin D examinations has been published by an expert working group of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) and the International Osteoporosis Foundation (IOF).
The publication has appeared in the journal Clinical Chemistry and Laboratory Medicine (CCLM).
The exploration of the metabolites in the degradation pathways of vitamin D has gained importance in recent years and simultaneous quantitation of 25-hydroxy vitamin D (25(OH)D) with 24,25-dihydroxyvitamin D (24,25(OH)2-D) has been proposed as a newer approach to define vitamin D deficiency. Yet, to date, no data are available on 24,25(OH)2-D biological variation (BV).
In the study, the Working Group evaluated the biological variation of 24,25(OH)2-D on the European Biological Variation Study (EuBIVAS) cohort samples to determine if analytical performance specifications (APS) for 24,25(OH)2-D could be generated. The study involved six European laboratories which recruited 91 healthy participants.
25(OH)D and 24,25(OH)2-D concentrations in EDTA plasma were examined weekly for up to 10 weeks in duplicate with a validated liquid chromatography coupled with mass spectrometers (LC-MS/MS) method. The Vitamin D Metabolite Ratio 24,25(OH)2-D divided by 25(OH)D × 100 was also calculated at each time point.
The findings were that the linear regression of the mean 24,25(OH)2-D concentrations at each blood collection showed that participants were not in steady state. Variations of 24,25(OH)2-D over time were significantly positively associated with the slopes of 25(OH)D concentrations over time and the concentration of 25(OH)D of the participants at inclusion, and negatively associated with body mass index (BMI).
The variation of the 24,25(OH)2-D concentration in participants over a period of 10 weeks was 34.6%. Methods that would detect a significant change linked to the natural production of 24,25(OH)2-D over this period would need a relative measurement uncertainty (MU%) < 14.9%, while at p<0.01, MU should be <10.5%.
Professor Etienne Cavalier, Professor of Clinical Chemistry at the University of Liège, Belgium and Chairman of the IFCC-IOF Committee for Bone Metabolism, stated, "This study expands our understanding of the degradation pathways of vitamin D in healthy individuals and, importantly, defines for the first time the analytical performance specifications for 24,25(OH)2-D examinations."
"Given the growing interest in this metabolite, some laboratories or manufacturers may aim to develop specific methods for its determination, and these findings are thus necessary prerequisites for their validation."
Professor Nicholas Harvey, Director of the MRC Lifecourse Epidemiology Centre, University of Southampton and Chair of the IOF Committee of Scientific Advisors, thanked the Working Group, stating, "This study demonstrates the huge value of international collaboration, bringing together key expertise from IFCC and IOF, and has established important technical parameters which will facilitate clinical validation of 24,25 dihydroxyvitamin D measurement, and potentially lead to more accurate assessment of vitamin D deficiency."
More information: Etienne Cavalier et al, Analytical performance specifications for the measurement uncertainty of 24,25-dihydroxyvitamin D examinations, Clinical Chemistry and Laboratory Medicine (CCLM) (2023). DOI: 10.1515/cclm-2023-0176


















