This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Gene p16, which drives colorectal cancer, is emerging as a target for potential therapies
Colorectal cancer is the fourth most common and second deadliest cancer. How colorectal cancer develops is not well understood, but a team led by researchers at Baylor College of Medicine reports in the Journal of Experimental & Clinical Cancer Research that silencing the gene p16, even though the DNA itself does not change, can drive colorectal cancer progression in animal models.
The researchers also revealed a strategy that reduced tumor growth and improved survival in tumor-bearing mice, opening new possibilities for future targeted therapies in patients with gene p16 alterations.
"Years of research have shown that human colorectal cancer occurs in steps," said corresponding author Dr. Lanlan Shen, professor of pediatrics—nutrition and member of the Dan L Duncan Comprehensive Cancer Center at Baylor.
"First, a gene mutates leading to the formation of benign polyps, small clumps of cells that grow in the lining of the colon. Then, changes in a second gene drive the benign polyps to become malignant or cancerous growth. This process has not been studied well because, until now, we did not have an animal model that recapitulated the human cancer."
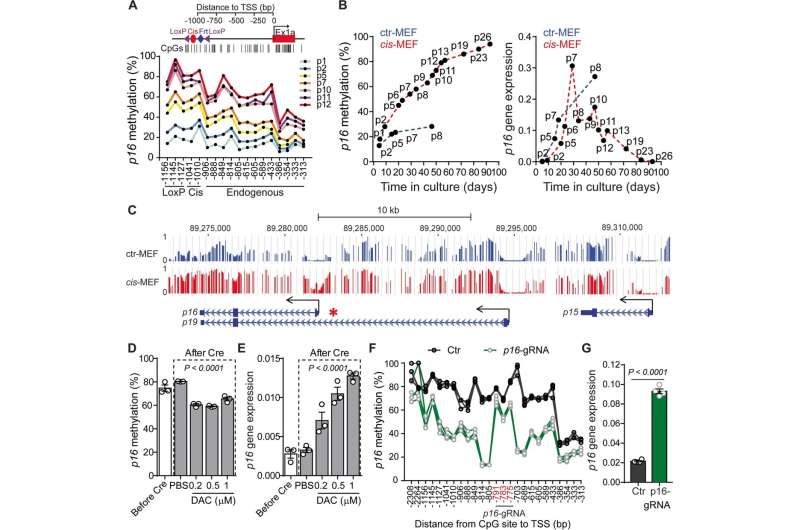
In this study, the Shen lab and colleagues developed the first animal model of colorectal cancer that mimics the developmental process of the human condition. Using this model, the team investigated a particular change in the second gene called p16 and its effect in colorectal cancer growth.
Normally, gene p16 suppresses tumor growth, but as people age, p16 is silenced. Aging is the strongest risk factor for colorectal cancer. Silenced p16 is frequently found in colorectal cancer, which could explain why older people get this type of cancer more than younger people.
In this case, p16 is not silenced by a mutation of the DNA sequence of the gene itself, but by a different process called epimutation. Epimutations are chemical modifications such as attaching or removing a methyl chemical group in a gene. This chemical modification or methylation acts like a switch, turning p16 off when adding a methyl group and turning it on by removing the methyl group. This is important from the therapeutic point of view, because, unlike mutations which are difficult to reverse, epimutations are intrinsically reversible.
"Our animal model is the first to include both a genetic mutation called Apc that can initiate a benign tumor growth, and an epigenetic change, methylation of gene p16," Shen said. "We conducted long-term survival and microscopy analysis of tumor development and progression and found that mice carrying combined Apc mutation and p16 epimutation had significantly shortened survival and increased tumor growth compared to those with Apc mutation only."
Interestingly, colorectal tumors with the gene p16 turned off were growing in a microenvironment that suppressed the immune response against the tumor, favoring cancer growth. These observations prompted the researchers to test a combined treatment for these tumors.
"We combined a drug that inhibits DNA methylation of gene p16, turning it on, with another drug that removes the suppression on the immune response, allowing it to attack the tumor," Shen said. "The combined drug treatment was more effective for improving survival in tumor-bearing mice than each drug alone."
Research has shown that human colorectal cancers have many DNA methylation alterations, but the contributions of these modifications to tumor initiation and maintenance remain an important unmet need.
"We seek to better understand the mechanisms by which epimutations frequently observed in cancer cells contribute to cancer development and progression," Shen said. "Our animal model is a valuable laboratory tool to study these mechanisms and also to screen potential treatments that could later be developed as therapies for patients."
More information: Li Yang et al, Functional characterization of age-dependent p16 epimutation reveals biological drivers and therapeutic targets for colorectal cancer, Journal of Experimental & Clinical Cancer Research (2023). DOI: 10.1186/s13046-023-02689-y