This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cancer discoveries could enhance immunotherapy, breast cancer care
Two new discoveries from the Dudley lab at UVA Cancer Center highlight the different roles of blood vessels in solid tumors—and the findings could help prevent breast cancer from spreading and enhance the effectiveness of one of the most important new cancer treatments in many years.
In one new scientific paper, researcher Andrew C. Dudley, Ph.D., and his team report that the effectiveness of immunotherapy drugs called immune check blockade is enhanced when blood vessels are targeted in a specific way. (Immunotherapy enhances the power of the immune system to fight cancer and other diseases.) In another paper, published soon after, Dudley and collaborators outline a finding that could help prevent breast cancer from metastasizing, or spreading to other parts of the body.
"Blood vessels can act both as good guys and bad guys in a growing tumor. On one hand, blood vessels are a passageway that allows special immune cells to enter the tumor, where they will hopefully find cancer cells and kill them. On the other hand, blood vessels act as a highway for cancer cells to enter the bloodstream and spread to distant sites in the body," said Dudley, of the University of Virginia School of Medicine's Department of Microbiology, Immunology and Cancer Biology.
"Our work highlights the dual role that blood vessels can play in cancer that we hope will improve our understanding of how to best elicit anti-tumor immune responses or even prevent metastasis."
Improving cancer immunotherapy
In the immunotherapy paper, Dudley and his team outline how the tumor vasculature—the blood vessels that feed a tumor—could be targeted to help immune cells enter the tumor and kill the cancer cells.
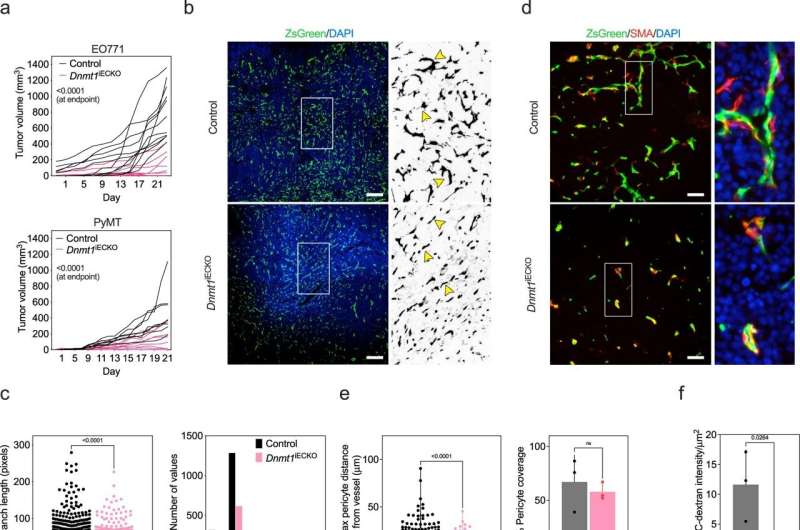
The researchers found that a particular enzyme, called DNMT1, is an important gatekeeper in blood vessels that determines whether anti-tumor immune cells can enter the tumor "microenvironment." When the investigators disabled this enzyme only in the blood vessels, they found it reduced vessel growth and made the vessels more receptive to immune cell entry; this led to an improved response to immunotherapies.
By blocking this enzyme with drugs, doctors might be able to bolster the numbers of anti-tumor immune cells that can enter patients' tumors and kill cancer cells. This approach could be combined with existing immunotherapy approaches to increase the number of patients who respond to the treatment, the researchers suggest.
"Blocking DNMT1 activity in cancer cells has recently been shown to bolster the efficacy of immunotherapies for cancer," said Dudley. "But we've found that DNMT1 is also targetable in cancer-associated blood vessels, and blocking its function there seems to be equally important."
Preventing metastasis
In the other paper, Dudley and his collaborators shed important new light on how breast cancer cells spread from the initial tumor site to other parts of the body, including the lymph nodes. These insights are particularly important because patients whose cancer spreads to the lymph nodes typically see worse outcomes—especially for breast cancer.
Dudley and his team found that highly metastatic breast cancer cells activate other types of cells, called fibroblasts, to "remodel" the surrounding environment so that cancer cells can escape the tumor, enter the lymphatic vessels, and spread to the lymph node. The researchers say that using drugs to interrupt this process early on could offer a way to prevent breast cancer from spreading.
"We've learned from this study how cancer cells can take advantage of non-cancerous cells in their surroundings and change their behavior in order to enter vessels and spread to different sites in the body—especially the lymph nodes," Dudley said. "Cancer really is a devious disease, and this is just one example of why it can be so difficult to treat. Therapies that target both the cancer cells and the non-cancerous cells that help the tumor along may prove beneficial in the long run."
Dudley hopes his lab's new findings will help advance the battle against cancer, a key mission of UVA Cancer Center, which has been designated one of only 54 Comprehensive Cancer Centers in the country by the National Cancer Institute. The NCI's Comprehensive Cancer Center designation recognizes elite cancer centers with the most exceptional cancer programs in the nation. Comprehensive Cancer Centers must meet rigorous standards for innovative research and leading-edge clinical trials, positioning them at the leading edge of cancer care.
"Cancer doesn't exist in a vacuum," Dudley said. "In other words, cancer cells have this whole ecosystem or microenvironment where they thrive. We hope that by understanding how cancer cells exploit their microenvironment, we can perhaps develop new types of therapies, improve existing drug responses or even prevent cancer from spreading."
Findings published
Dudley and his collaborators have published their immune checkpoint blockade findings in Nature Communications. The team consisted of Dae Joong Kim, Swetha Anandh, Jamie L. Null, Piotr Przanowski, Sanchita Bhatnagar, Pankaj Kumar, Sarah E. Shelton, Erin E. Grundy, Katherine B. Chiappinelli, Roger D. Kamm, David A. Barbie and Dudley. Dudley reported no financial interest in the work; a full list of the other authors' disclosures is included in the paper.
The metastasis findings have been published in the journal Cancer Research. That team consisted of Null, Kim, James V. McCann, Patcharin Pramoonjago, Jay W. Fox, Kumar, Lincy Edatt, Chad V. Pecot and Dudley.
More information: Dae Joong Kim et al, Priming a vascular-selective cytokine response permits CD8+ T-cell entry into tumors, Nature Communications (2023). DOI: 10.1038/s41467-023-37807-z
Jamie L. Null et al, Periostin+ stromal cells guide lymphovascular invasion by cancer cells, Cancer Research (2023). DOI: 10.1158/0008-5472.CAN-22-2412