This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chronic stress-related neurons identified
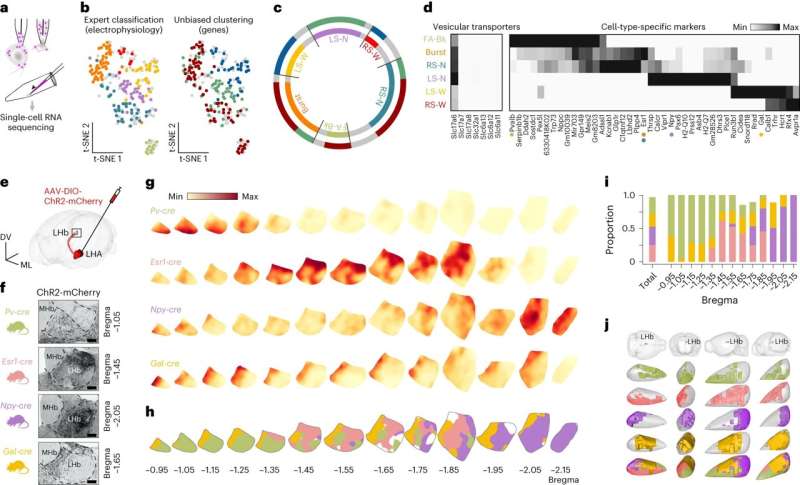
Researchers at Karolinska Institutet in Sweden have identified a group of nerve cells in the mouse brain that are involved in creating negative emotional states and chronic stress. The neurons, which have been mapped with a combination of advanced techniques, also have receptors for estrogen, which could explain why women as a group are more sensitive to stress than men. The study is published in Nature Neuroscience.
Just which networks in the brain give rise to negative emotions (aversion) and chronic stress have long been unknown to science.
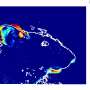
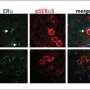
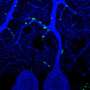
By using a combination of advanced techniques, such as Patch-seq, large-scale electrophysiology (Neuropixels) and optogenetics, KI researchers Konstantinos Meletis and Marie Carlén and their team have been able to map out a specific neuronal pathway in the mouse brain leading from the hypothalamus to the habenula that controls aversion.
The researchers used optogenetics to activate the pathway when the mice entered a particular room, and found that the mice soon started to avoid the room even though there was nothing in it.
Opens the way for novel treatments for depression
"We discovered this connection between the hypothalamus and the habenula in a previous study but didn't know what types of neurons the pathway was made up of," says Konstantinos Meletis, professor at the Department of Neuroscience, Karolinska Institutet. "It's incredibly exciting to now understand what type of neuron in the pathway controls aversion. If we can understand how negative signals in the brain are created, we can also find mechanisms behind affective diseases like depression, which will open the way for novel drug treatments."
The study was led by three postdocs at the same department, Daniela Calvigioni, Janos Fuzik and Pierre Le Merre, and as Professor Meletis explains, is an example of how scientists can use advanced techniques to identify neuronal pathways and neurons that control emotions and behavior.
Sensitive to estrogen levels
Another interesting discovery is that the neurons linked to aversion have a receptor for estrogen, making them sensitive to estrogen levels. When male and female mice were subjected to the same type of unpredictable mild aversive events, the female mouse developed a much more lasting stress response than the male.
"It has long been known that anxiety and depression are more common in women than in men, but there hasn't been any biological mechanism to explain it," says Marie Carlén, professor at the Department of Neuroscience. "We've now found a mechanism that can at least explain these sex differences in mice."
More information: Le Merre, P. et al. Esr1+ hypothalamic-habenula neurons shape aversive states, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01367-8. www.nature.com/articles/s41593-023-01367-8