This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Next-generation trial shows five-drug combo can keep 'ultra-high-risk' bone marrow cancer at bay
Combining five existing drugs can keep a highly aggressive type of bone marrow cancer at bay for longer, an innovative trial has shown.
The new five-drug cocktail, along with a stem cell transplant, kept the cancer at bay for two and a half years for more than three quarters of "ultra-high-risk" myeloma patients. In comparison, less than half of patients on the current standard of care lived for that long without seeing their cancer progress.
The MUK Nine OPTIMUM trial, co-led by The Institute of Cancer Research, London, the University of Leeds and The Royal Marsden NHS Foundation Trust, is a first of its kind in myeloma—using sophisticated statistical analysis to compare results with those from an earlier trial, instead of a control group.
This new "digital comparator" approach allows researchers to fast-track comparative evidence, helping them obtain answers quickly while also lowering trial costs.
The five drugs—bortezomib, lenalidomide, daratumumab, dexamethasone and cyclophosphamide chemotherapy—are individually licensed and in clinical use, which means the combination has the potential to become available to patients relatively quickly if it is recommended by regulatory bodies.
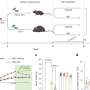
Researchers at The Institute of Cancer Research (ICR) and University of Leeds compared outcomes from 103 ultra-high-risk patients in the OPTIMUM trial treated with the five-drug cocktail and 117 ultra-high-risk patients in a separate trial known as Myeloma XI (MyXI), who were treated with the current standard of care: a different combination of four drugs—carfilzomib, lenalidomide, cyclophosphamide and dexamethasone. The latest findings are published in the Journal of Clinical Oncology.
Ultra-high-risk myelomas relapse more quickly
Ultra-high-risk myelomas have certain genetic changes that make them more aggressive, less responsive to treatment and likely to relapse more quickly. These patients face particularly poor outcomes and are at very high risk of relapsing within the first two years from diagnosis, compared with others without the genetic changes who tend to stay in remission for five years or longer.
Researchers showed that ultra-high-risk patients on the OPTIMUM trial who received the five-drug combination were more likely than MyXI patients on the standard treatment to stay in remission for longer before seeing their disease progress and symptoms worsen.
At 30 months, three-quarters (77%) of OPTIMUM patients had stable disease unlikely to progress in the immediate future, compared with less than half (39.8%) of MyXI patients.
At 30 months follow-up, 83.5% of patients on the OPTIMUM trial who received the five-drug combination were still alive, compared with 73.5% of those on the MyXI trial.
The results of the trial show that using the intensified treatment approach consisting of five drugs could be a promising strategy for ultra-high-risk patients—highlighting the importance of molecularly profiling patients to identify those with the high-risk form of the disease, so that they can receive more intense treatment early on.
Finding a more tailored approach
Study leader Dr. Martin Kaiser, Team Leader in Myeloma Molecular Therapy at The Institute of Cancer Research, London, and Consultant Hematologist at The Royal Marsden NHS Foundation Trust, said, "Our aim is to find the best initial treatment for people with ultra-high-risk myeloma, to hopefully improve their outcomes. We have shown that a more tailored approach involving five different drugs could help treat patients with the highest-risk forms of myeloma—helping keep them alive and healthy for longer.
"We hope the NHS will now consider our findings from this trial which was designed together with patients, so that this combination can become available to high-risk myeloma patients in the U.K. and elsewhere.
"As a next generation 'digital comparator' trial, the OPTIMUM trial also sets a new standard for fast-tracking evidence. It is a successful proof of principle study which could help inform and speed up future studies."
More information: Martin F. Kaiser et al, Daratumumab, Cyclophosphamide, Bortezomib, Lenalidomide, and Dexamethasone as Induction and Extended Consolidation Improves Outcome in Ultra-High-Risk Multiple Myeloma, Journal of Clinical Oncology (2023). DOI: 10.1200/JCO.22.02567