This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
New PET imaging model for quantifying cardiac amyloidosis can enable more accurate diagnosis and monitoring
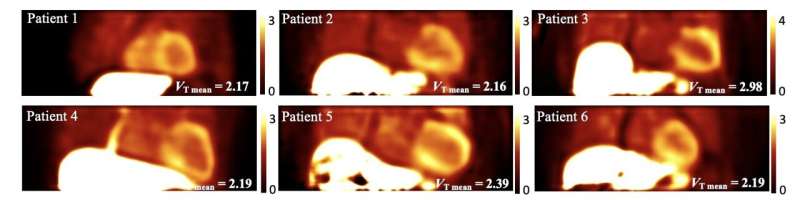
Researchers have identified the optimal tracer kinetic model to quantify 18F-flutemetamol myocardial uptake in patients with transthyretin (ATTR) cardiac amyloidosis. Presented at the 2023 Society of Nuclear Medicine and Molecular Imaging Annual Meeting, this method allows physicians to obtain a fully quantitative measurement of cardiac amyloidosis burden and response to treatment for the first time.
ATTR cardiac amyloidosis is a rare, life-threatening disorder caused by the buildup of abnormal proteins, called amyloid plaques, in the heart tissue. As the proteins build up, it becomes harder for the heart to function properly and can eventually lead to heart failure.
Recent studies have shown the promise of 18F-flutemetamol PET imaging for detecting ATTR cardiac amyloidosis. "Static PET data analysis methods for 18F-flutemetamol are time sensitive, due to patient-dependent tracer washout from the myocardium. As a result, these methods can only provide physicians with a limited read-out," stated Qiong Liu, a Ph.D. student at Yale University in New Haven, Connecticut. "New methods to obtain fully quantitative myocardial volume of distribution with this tracer are needed."
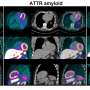
To determine the optimal method for quantifying 18F-flutemetamol myocardial uptake, Liu and colleagues performed kinetic modeling on dynamic 18F-flutemetamol PET scans of six ATTR cardiac amyloidosis patients. Dynamic images were reconstructed, and several compartmental models were tested. Parametric images of the volume of distribution were then generated and analyzed.
Researchers found that the kinetics of 18F-flutemetamol are best quantified using a two-tissue reversible compartment model. The simplified graphical Logan plot identified the volume of distribution parameter generated from the first 30-minute dynamic scan as a quantitative parameter that is potentially useful for diagnosing and evaluating treatment in patients with ATTR cardiac amyloidosis.
"These findings suggest that commonly employed semiquantitative methodologies, such as standardized uptake values used for 18F-FDG, are not optimal for 18F-flutemetamol. Using parametric modeling to fully quantify volume of distribution may provide clinicians with a more accurate and robust method for assessing the presence and progression of amyloid plaques in the myocardium, ultimately improving patient care," said Liu.
The study underscores the importance of understanding the fundamental kinetics of tracers in the target organ of interest, which is especially important to the optimal use of any new tracer. Similar approaches using dynamic and parametric imaging may also be beneficial for other tracers.
More information: Abstract 270. "Dynamic Imaging and Tracer Kinetic Modeling of 18F-flutemetamol PET for ATTR Cardiac Amyloidosis Patients." Link to Session