June 2, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Wharton's jelly found to halt diabetes progression in Phase II trial
Research in Sweden led by the University of Uppsala has looked into using mesenchymal stromal cells to halt the progress of newly diagnosed type 1 diabetes. In the paper, "Umbilical cord-derived mesenchymal stromal cells preserve endogenous insulin production in type 1 diabetes: a Phase I/II randomized double-blind placebo-controlled trial," published in the journal Diabetologia, researchers detail the study and results of a small sample set of 24 participants.
The researchers used Wharton's jelly-derived mesenchymal stromal cells (MSCs) that were not derived from the patients in the study (allogeneic).
Wharton's jelly is the gelatinous mucus-like tissue surrounding the umbilical cord's blood supply between a developing fetus and the placenta. The jelly-derived MSCs have regenerative potential, limited cell type differentiation, self-renewal properties, and signaling with other cells resulting in immunomodulation. The immunomodulatory properties of these MSCs allow them to be used in allogeneic "off-the-shelf" therapies as the body does not reject them as foreign tissue.
A selection process called ProTrans was applied in collecting the MSCs utilized. The ProTrans selection process was created by NextCell, a Swedish biopharmaceutical company associated with several researchers involved in the study.
The researchers conducted a combined Phase I/II trial, composed of a dose escalation followed by a randomized, double-blind, placebo-controlled study. Treatment with ProTrans allogeneic MSCs was compared with a placebo in adults with newly diagnosed type 1 diabetes.
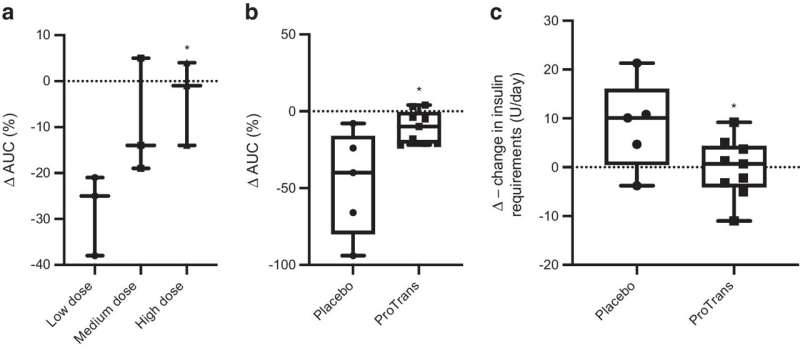
The study was designed to test changes in C-peptide for a mixed meal tolerance test at one year following ProTrans/placebo infusion compared with baseline performance before treatment.
C-peptide levels in placebo-treated individuals declined by 47%, whereas those in ProTrans-treated individuals declined by only 10%. Similarly, insulin requirements increased in placebo-treated individuals by a median of 10 U/day, whereas insulin needs of ProTrans-treated individuals did not change over the follow-up period of 12 months.
This study suggests that allogeneic Wharton's jelly-derived MSCs via ProTrans is a safe treatment for recent-onset type 1 diabetes, with the potential to preserve beta cell function long term.
No other treatments on the market or in clinical trials have shown the ability to halt the progression of diabetes.
Double-blinding
A double-blinded study is designed so that participants and researchers have no idea if a potential treatment or placebo is being administered. While double-blinded studies help remove experimental bias, they may be considered critical to the credibility of results when researchers, or the research being conducted, are connected to a company with a financial interest in the outcome. The current study design gives an excellent example of steps that can be taken to ensure certain forms of bias are eliminated.
Randomization was performed with a web-based randomization code created prior to the start of the study, with participants randomized to ProTrans or placebo treatment. Envelopes containing matching code assignments were kept in a locked room at the clinic, with study staff opening the envelopes at the baseline visits. In this way, the participants were already randomized before meeting the study staff, and no assignment bias could occur.
All participants and study personnel were blinded to group assignments and treatment type. Only after all data was collected and analyzed was the randomization decoded and the results reviewed by Medical Statistics Unit, Karolinska Instituet.
More information: Per-Ola Carlsson et al, Umbilical cord-derived mesenchymal stromal cells preserve endogenous insulin production in type 1 diabetes: a Phase I/II randomised double-blind placebo-controlled trial, Diabetologia (2023). DOI: 10.1007/s00125-023-05934-3
© 2023 Science X Network