This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Evaluating cetuximab's effectiveness and toxicity in advanced cutaneous squamous cell skin cancer
A new research paper was published in Oncotarget, titled, "Effectiveness and toxicity of cetuximab with concurrent RT in locally advanced cutaneous squamous cell skin cancer: a case series."
Treatment for locally advanced cutaneous squamous cell cancers (laCSCC) remains poorly defined. Most laCSCC tumors express high levels of epidermal growth factor receptors (EGFR). Cetuximab has activity in other EGFR expressing cancers and enhances the effectiveness of radiotherapy.
In this new study, researchers Mark Chang, Wolfram Samlowski and Raul Meoz from University Medical Center of Southern Nevada, Comprehensive Cancer Centers of Nevada, University of Nevada Las Vegas, and University of Nevada Reno conducted a retrospective review of institutional data and identified 18 patients with laCSCC treated with cetuximab induction and concurrent radiotherapy.
"We performed a retrospectively review of treatment outcome and toxicity in our patients who received concurrent cetuximab and radiotherapy to show an additional potentially effective treatment option for patients with laCSCC. The goal is also to provide data to inform the design of potential prospective clinical trials," say the researchers.
The loading dose of cetuximab was 400 mg/m² IV. Subsequent weekly doses of 250 mg/m² IV were infused throughout the period of radiation. The treatment doses ranged from 4,500–7,000 cGy, with a dose fraction of 200–250 cGy. The objective response rate was 83.2% with 55.5% complete responses and 27.7% partial responses.
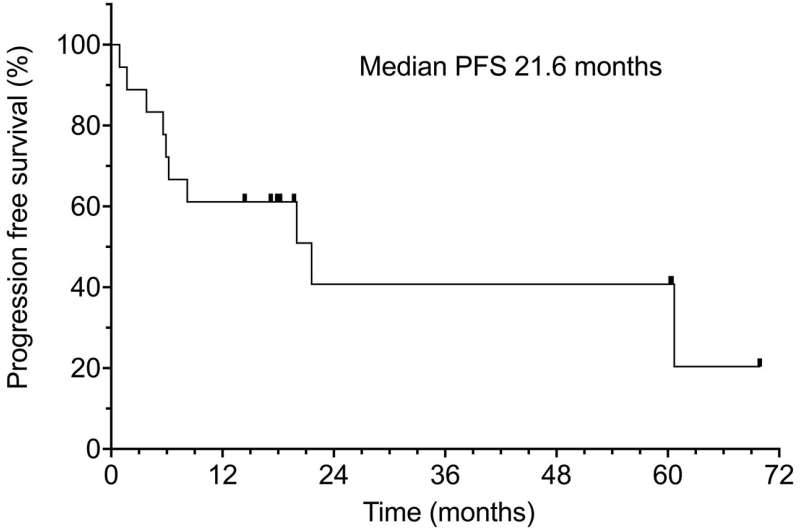
Median progression-free survival was 21.6 months. Progression-free survival was 61% at one year and 40% at two years. With longer follow-up, some patients developed a local recurrence (16.7%), distant metastases (11.1%) or a second primary cancer (16.3%). Cetuximab was well tolerated, with 68.4% patients experienced only mild acneiform skin rash or fatigue (Grade 1 or 2). Radiotherapy produced expected side effects (skin erythema, moist desquamation, mucositis).
"Cetuximab plus radiotherapy represents an active and tolerable treatment option for laCSCC, including patients with contraindications for checkpoint inhibitor therapy," conclude the researchers.
More information: Mark Chang et al, Effectiveness and toxicity of cetuximab with concurrent RT in locally advanced cutaneous squamous cell skin cancer: a case series, Oncotarget (2023). DOI: 10.18632/oncotarget.28470