This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers identify and edit gene mutation implicated in familial heart disease
Dilated cardiomyopathy (DCM) is one of the most common causes of heart failure, affecting nearly 1 in 400 people worldwide. About 40%–50% of DCM cases are estimated to have an identifiable genetic cause, which explains why the disorder often runs in families.
In a pair of new studies published in Nature Communications, researchers from EMBL Heidelberg's Steinmetz group have explored the molecular underpinnings of a particular form of familial DCM and tested gene-editing approaches for treating such disorders.
About 3% of familial DCM cases involve mutations in a crucial gene called RBM20. This gene, which helps regulate a type of RNA processing called "alternative splicing," is predominantly expressed in cardiomyocytes, i.e., heart muscle cells. In the first of the two studies, the Steinmetz group explored the molecular pathways by which mutations in RBM20 affect cardiomyocyte function.
When proteins lose their way
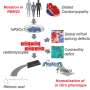
A defect in the genetic code may cause disease in different ways. In certain mutations, disease characteristics (or the "phenotype") arise due to the gene's normal function being disrupted by the mutation. If one thinks of the cell as a complex machine, the mutated gene can be considered a broken gear that slows down or halts normal function. For a long time, scientists believed this to be the main effect of RBM20 mutations—it prevented the gene from performing its normal role in RNA splicing, in turn affecting cellular function.
However, as the Steinmetz group explored in their study, the disease phenotype can also be caused by a protein behaving spuriously, causing damage by doing what it's not supposed to do or being where it's not supposed to be. In the analogy above, one could think of it as a faulty part leaking oil, which affects the other parts of the machine and messes up the entire system.
In agreement with previous studies, the team showed that a mutation in the gene can cause the RBM20 protein to localize to the cytoplasm of the cell instead of the nucleus where it is normally found. While in the cytoplasm, RBM20 can form detrimental cytoplasmic granules that worsen disease phenotype, similar to other diseases involving protein mislocalization, such as neurodegenerative diseases and cancer.
"This distinction can affect how one approaches therapeutics," said Julia Kornienko, Ph.D. student in the Steinmetz lab and the first author of the study. "While, for a loss-of-function mutation, a viable strategy would be to supply more healthy RBM20 to the cell to rescue the RNA splicing defect, this would not work for mislocalized RBM20 since it would have no impact on the localization."
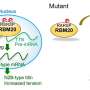
The team then used a genome-wide CRISPR-Cas9 screen and image-enabled cell sorting (ICS)—a next-generation cell sorting method advanced by the Steinmetz lab in collaboration with BD Biosciences and other researchers at EMBL—to tease out RBM20's molecular interactors. Through this process, they identified a protein—TNPO3—as being responsible for transporting RBM20 from the cytoplasm to the nucleus. They also found that mutations in RBM20 can prevent this interaction, resulting in RBM20 accumulating in the cytoplasm.
"Achieving genome-wide scale without the ICS technology would have been very challenging, if at all possible," noted Kornienko. "In addition, an arrayed screen, even for a selected subset of the genome, would have taken a substantially longer time."
When the researchers overexpressed TNPO3 in cells, it enhanced the nuclear import of RBM20 and reversed the RNA splicing defects caused by RBM20 mutations. This finding has implications for developing novel therapeutic approaches targeted at restoring nuclear localization of RBM20, which the team is currently further investigating.
"This is a novel interaction that had never been described before," said Kornienko. "At the moment, we are working on deciphering the structure of the RBM20–TNPO3 complex in the lab. Knowing the exact structure of the complex will allow us to identify small molecule drugs that stabilize the interaction upon mutation and select a compound library to test for therapeutic effects in vitro or in vivo. We are also exploring other ways of restoring RBM20's nuclear localization in vivo. We are very excited about this conceptually novel approach to target RBM20 in DCM and hope it could improve therapy options for RBM20 patients in future."
Targeted gene editing to the rescue
In the second study, the researchers took a more direct approach towards reversing RBM20-caused cardiomyocyte dysfunction in mouse models of DCM.
Current therapeutic strategies for DCM are severely limited in their effectiveness and accessibility, and often a heart transplant is the only option for advanced patients. Ever since the discovery of the CRISPR-Cas9 gene editing system, scientists have been exploring its potential in the treatment of human diseases caused by mutations in specific genes. Many such approaches depend on processes active in dividing cells, thus limiting their applicability for mature cells that do not divide, e.g., cardiomyocytes or nerve cells.
The Steinmetz lab used a next-generation CRISPR-based system called base editing that can be used in such post-mitotic cells to selectively reverse mutations in RBM20 in cardiomyocytes. To do this, they injected mice carrying Rbm20 mutations with a specially created viral vector that selectively targets cardiomyocytes and that carries a gene-delivery system that allows the genetic code of these cells to be edited at the site of the mutation.
Within three months of this treatment, the researchers saw improvement in several disease phenotypes, such as heart pumping function, splicing deficiency, and expression of heart failure biomarkers. They estimate that they could reverse the mutation in almost 70% of the cardiomyocytes in the recipient mice, an impressive figure for therapies of this kind. Interestingly, the gene editing also reversed cytoplasmic mislocalization of the RBM20 protein in these mice. Importantly, the researchers did not see any off-target editing in other parts of the body—the biggest reservation for transferring CRISPR-Cas9 editing approaches into clinics.
"Getting this method to work in mouse models was quite challenging," said Markus Grosch, postdoc in the Steinmetz lab and first author of this study. "By increasing the amount of the virus, decreasing the age (and consequently the weight) of the mice, and using cardiomyocyte-specific viral delivery, we could dramatically increase the editing efficacy."
The researchers believe that in the future, in addition to serving as a possible treatment for DCM patients, such a strategy could also have potential as a preventative measure for those at very early stages of the disease or those who carry similar genetic mutations but have not yet developed DCM.
"Our team aims to explore genome-editing strategies to understand the genetic basis of complex phenotypes, and in doing so, transform the way we do biomedical research," said Lars Steinmetz, Dieter Schwarz Foundation Professor and Associate Group Leader at EMBL Heidelberg. "DCM is one of the leading causes of heart failure and despite major advances in medicine, there are no targeted therapies available to patients. Studies like ours delve into the biology behind the disease to identify new therapeutic avenues and strategies to tackle biomedical challenges in the future."
More information: Julia Kornienko et al, Mislocalization of pathogenic RBM20 variants in dilated cardiomyopathy is caused by loss-of-interaction with Transportin-3, Nature Communications (2023). DOI: 10.1038/s41467-023-39965-6
Markus Grosch et al, Striated muscle-specific base editing enables correction of mutations causing dilated cardiomyopathy, Nature Communications (2023). DOI: 10.1038/s41467-023-39352-1