This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
A multiplex assay to assess activated p300/CBP in circulating prostate tumor cells
A new research paper was published in Oncotarget, titled, "Development of a multiplex assay to assess activated p300/CBP in circulating prostate tumor cells."
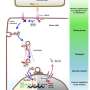
Reduced SIRT2 deacetylation and increased p300 acetylation activity leads to a concerted mechanism of hyperacetylation at specific histone lysine sites (H3K9, H3K14, and H3K18) in castration-resistant prostate cancer (CRPC). In this new study, researchers Mikolaj Filon, Bing Yang, Tanaya A. Purohit, Jennifer Schehr, Anupama Singh, Marcelo Bigarella, Peter Lewis, John Denu, Joshua Lang, and David F. Jarrard from the University of Wisconsin examined whether circulating tumor cells (CTCs) identify patients with altered p300/CBP acetylation.
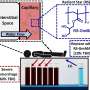
"In the current study, employing a novel Exclusion-based Sample Preparation (ESP) technology (9) to isolate CTCs, we evaluated p300 activity, SIRT2 expression and H3K18 acetylation in CTCs in a series of patients with sensitivity or resistance to ADT," say the researchers.
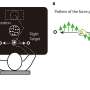
CTCs were isolated from 13 advanced PC patients using Exclusion-based Sample Preparation (ESP) technology. Bound cells underwent immunofluorescent staining for histone modifying enzymes (HMEs) of interest and image capture with NIS-Elements software. Using the cBioPortal PCF/SU2C dataset, the response of CRPC to androgen receptor signaling inhibitors (ARSI) was analyzed in 50 subjects.
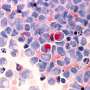
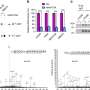
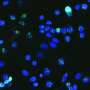
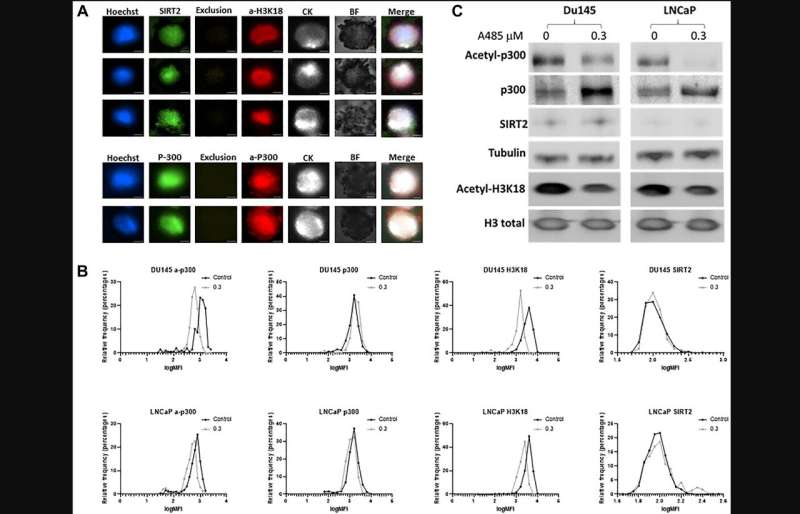
Staining optimization and specificity revealed clear expression of acetyl-p300, acetyl-H3K18, and SIRT2 on CTCs (CK positive, CD45 negative cells). Exposure to A-485, a selective p300/CBP catalytic inhibitor, reduced p300 and H3K18 acetylation. In CRPC patients, a-p300 strongly correlated with its target acetylated H3k18 (Pearson's R = 0.61), and SIRT2 expression showed robust negative correlation with a-H3k18 (R = −0.60).
A subgroup of CRPC patients (6/11; 55%) demonstrated consistent upregulation of acetylation based on these markers. To examine the clinical impact of upregulation of the CBP/p300 axis, CRPC patients with reduced deacetylase SIRT2 expression demonstrate shorter response times to ARSI therapy (5.9 vs. 12 mo; p = 0.03). A subset of CRPC patients demonstrate increased p300/CBP activity based on a novel CTC biomarker assay.
"With further development, this biomarker suite may be used to identify candidates for CBP/p300 acetylation inhibitors in clinical development," conclude the researchers.
More information: Mikolaj Filon et al, Development of a multiplex assay to assess activated p300/CBP in circulating prostate tumor cells, Oncotarget (2023). DOI: 10.18632/oncotarget.28477