This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Existing cancer drugs have potential to benefit thousands more patients, research shows
New research, published in Nature Genetics, shows that more people may benefit from PARP inhibitor drugs, like olaparib, which are already used to treat some breast, ovarian and prostate cancer patients who have inherited an altered BRCA1 or BRCA2 gene—often known as the "Jolie genes."
The findings suggest PARP inhibitors may also be effective for patients with cancers which have changes in the SF3B1 gene which is involved in processing genetic information that is used to build proteins.
The team at The Institute of Cancer Research, London, investigated changes in the SF3B1 gene, which are linked to several cancer types, including some estrogen receptor positive (ER-positive) breast cancers and some types of leukemia and melanoma.
Researchers performed a large-scale assessment of 80 drugs that are either already in use for cancer patients or are in late-stage development to find those which might be effective against cancers with an altered SF3B1 gene.
PARP inhibitors effective against cancers with altered SF3B1 gene
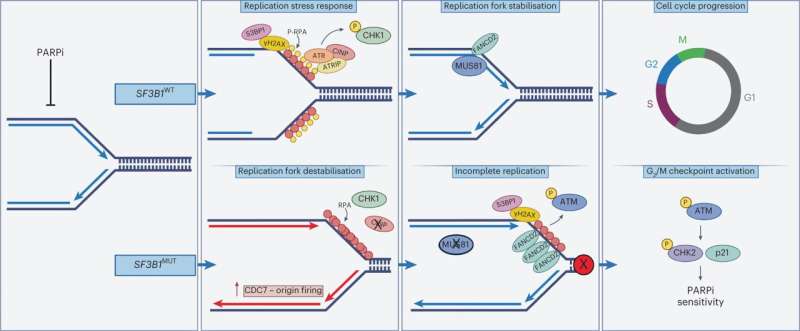
The researchers discovered that PARP inhibitor drugs reduced the ability of cancer cells with an altered SF3B1 gene to survive.
One of the ways PARP inhibitors work in cancer cells with changes in their BRCA genes is by preventing them from repairing their DNA, leading to a build-up of damage and causing the cells to die.
This happens in cells with changes in the BRCA genes because they lack an important type of DNA repair mechanism, which forces them to rely on the PARP protein to repair the DNA. The PARP inhibitor drugs sabotage this process by trapping the PARP protein onto the DNA, causing even more damage, which kills the cancer cell.
For the first time, the scientists at the Breast Cancer Now Toby Robins Research Center at The Institute of Cancer Research discovered that even when cancer cells with the altered SF3B1 gene have normal BRCA genes and therefore back-up DNA repair options, they were still weakened by PARP inhibitors.
They showed this is because cells with an altered SF3B1 gene also lack a protein called CINP, which is important for regulating the cells' response to PARP inhibitors. Without CINP, cancer cells aren't able to properly copy their DNA when treated with the drug. This causes a build-up of defective DNA, and the cells stop growing and die.
The team also looked at how the PARP inhibitor drug talazoparib affected mice with uveal melanoma and leukemia tumors with the altered SF3B1 gene. They found the drug stopped the growth of existing tumors and prevented the cancer spreading to other organs.
Existing drugs could help thousands more patients
If further research supports these findings, there is the potential that existing PARP inhibitor drugs such as olaparib, talazoparib and niraparib, might be able to help thousands more patients in the future.
Estimates show that SF3B1 gene changes affect around 3% of women with primary breast cancers and around seven percent of those with incurable secondary (metastatic) breast cancer. They can occur in up to 20% of patients with some types of melanoma that affect the eye and also leukemia.
Dr. Rachael Natrajan, functional genomics team leader at The Institute of Cancer Research, London, said, "PARP inhibitors are targeted medicines which are known for their effectiveness against cancers caused by inherited mutations in the BRCA genes.
"Our exciting findings show that PARP inhibitors can also exploit a weakness in cancer cells which have mutations in the SF3B1 gene and suggest there may be a whole new group of patients, including those with estrogen receptor positive breast cancer—the most common type of breast cancer—who could benefit from this personalized medicine."
Findings to be taken forward in clinical trials
Dr. Phil Bland, the first author and postdoctoral training fellow in the Breast Cancer Now Toby Robins Research Center at The Institute of Cancer Research, London, said, "Our study presents a huge shift in our understanding of the role of SF3B1 gene changes in cancer cells, potentially meaning that many more cancer patients could benefit from existing treatments."
"As PARP inhibitors are already approved for use for some patients with changes in their BRCA1 or BRCA2 genes, we hope to soon begin a clinical trial to establish their benefit in a wider group of cancer patients. If these are successful, we hope to see PARP inhibitors made available to more patients relatively quickly."
Dr. Simon Vincent, director of research, support and influencing at Breast Cancer Now, said, "PARP inhibitor drugs have been shown to save the lives of women with certain types of early breast cancer so it's incredibly exciting that even more patients could potentially benefit from them in the future, including those with the most common type of breast cancer.
"Further research is now needed to understand more about how they could help people with different types of cancer and possibly transform the way the disease is treated."
More information: Philip Bland et al, SF3B1 hotspot mutations confer sensitivity to PARP inhibition by eliciting a defective replication stress response, Nature Genetics (2023). DOI: 10.1038/s41588-023-01460-5