This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study connects neural gene expression differences to functional distinctions
Figuring out how hundreds of different kinds of brain cells develop from their unique expression of thousands of genes promises to not only advance understanding of how the brain works in health, but also what goes wrong in disease. A new MIT study that precisely probes this "molecular logic" in two neuron types of the Drosophila fruit fly, shows that even similar cells push and pull many levers to develop distinct functions.
In the study in Neuron, a team of neurobiologists at The Picower Institute for Learning and Memory found that the two closely related neuronal subtypes differed from each other in how they expressed more than 800 genes, or ~5% of the total genes encoded in the fly genome. By manipulating genes whose expression differed most prominently, the scientists were then able to show how they produced several of the observable differences between the cells.
"There is a global effort in neuroscience to identify all the different types of neurons to define their unique properties and their gene expression profiles," said study senior author Troy Littleton, Menicon Professor of Neuroscience in MIT's Departments of Biology and Brain and Cognitive Sciences. "That information can be used as a toolkit for studying how newly found disease genes map on to those particular neurons to indicate which ones might be most affected in specific brain disorders."
"We wanted to use Drosophila as a way to see whether we can, in fact, determine how the transcriptome of two similar neurons is differentially used to understand which key genes specify their unique structural and functional properties."
Under the microscope
The two neuron types compared in the study both emerge from the fly's analog of a spinal cord to control muscles by releasing the neurotransmitter glutamate at connections called synapses. The neurons' main functional differences are that "phasic" neurons connect to many muscles and emit big, occasional bursts of glutamate while "tonic" neurons each connect to only one muscle and provide more of a constant drip of the chemical. This duality, which is also found in neurons of the human brain, provides a flexible range of control.
Picower Institute postdoc Suresh Jetti led the effort in Littleton's lab to determine how these two neurons develop their differences. The team began with an unusually deep characterization of how the two cell types differ in form and function and then took a highly precise look at the gene expression profiles, or transcriptomes.
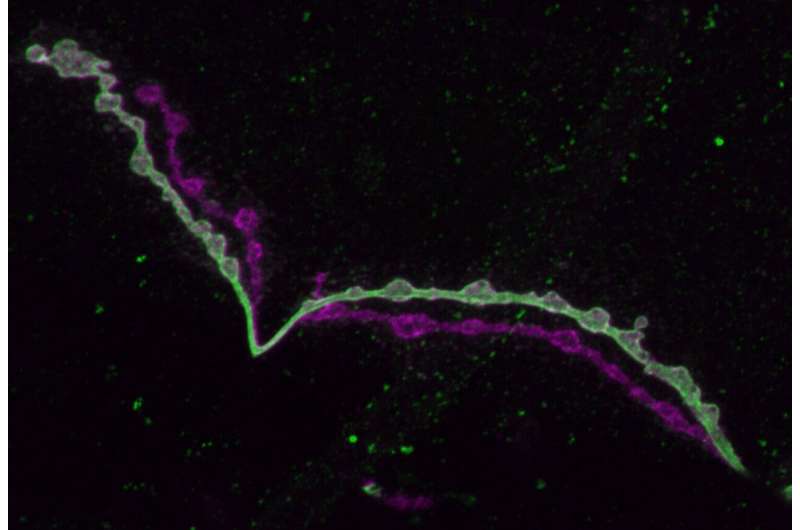
On close examination, the tonic and phasic cells showed a variety of important differences. Phasic neurons make fewer synapses on an individual muscle than tonic ones do, but because they innervate so many more muscles, phasic neurons have to make about four times as many synapses in total. The tonic neurons have more inputs from other neurons thanks to more widely reaching dendrites (the branches that lead into the cell).
On the output side of things, the phasic neurons produced much more powerful signals when stimulated and were more likely to send them than tonic neurons were. Analysis showed that the synaptic sites that prompt glutamate release, called active zones (AZs), took in more calcium ions in phasic neurons than tonic ones.
A particularly new and intriguing finding was that the AZs in tonic and phasic neurons took on different shapes. Tonic AZs were round, like donuts, while phasic ones were more triangular or star-shaped. Littleton hypothesizes that this difference could allow for more calcium ions to crowd into the phasic active zones, perhaps explaining their greater bursts of glutamate release compared to tonic neurons.
Expressing their differences
To assess gene expression, Jetti employed a technique called "isoform patchseq" in which he identified the exact same tonic and phasic neurons in hundreds of flies and extracted RNA from their individual nuclei and cell bodies. The technique, while very hard work, provided the team with an unusually rich vein of transcriptomic information from precisely the cells of interest, Littleton said, including not only how gene expression differed between the two cell types, but also how gene splicing and RNA editing were different.
In all, the expression of 822 genes was significantly different between the two neuron types. About 35 of the genes were known to help guide the growth of the axon branches that neurons extend to forge their connections with muscle—a set of differences pertinent to why tonic neurons innervate only one muscle while phasic ones innervate many.
Other differentially expressed genes related to the structure and function of synapses, while more than 20 others suggested differences in the neuromodulatory chemicals each neuron was sensitive to as inputs.
The team found that transport proteins were more prominently expressed in phasic neurons, perhaps explaining how they keep up with the greater demand to forge more synapses across many muscles. The team also found that while tonic neurons express "sialylation" genes to attach sugars to proteins on their synaptic membrane, phasic ones expressed unique "ubiquitin" genes that break down proteins.
After documenting which genes were most prominently different, the team set out to determine what they do by disrupting their function and seeing how that affected the cells.
For instance, Jetti, Littleton and colleagues found that interfering with specific ubiquitination genes caused phasic neurons to overgrow synapses. Disrupting sialylation, meanwhile, caused synaptic undergrowth in tonic neurons. Tonic neurons also expressed 40 times more of a gene called Wnt4, and disrupting Wnt4 reduced synaptic growth in this population of neurons.
The scientists had also found that phasic neurons express a calcium-ion buffering gene >30-fold more than tonic ones. When they mutated that gene to disrupt its function, they found that phasic neurons, which normally have lower baseline calcium levels, now display higher resting calcium similar to the tonic neurons.
And in another experiment they showed they could distinctly disrupt each cell's AZ shapes by interfering with cytoskeletal genes that each neuron expressed especially highly. When the team reduced a gene that phasic neurons express a lot, their AZs became elongated, but tonic AZs were unaffected. When the team reduced a gene that phasic neurons highly express, their AZs became less round without affecting AZs in phasic cells.
In all, the analysis enabled the team to begin constructing a model of the molecular differences that make the two cells differ, though Littleton said they still have more work to understand how the full repertoire of gene expression differences define the unique properties of the two neuronal subtypes.
More information: Suresh K. Jetti et al, Molecular logic of synaptic diversity between Drosophila tonic and phasic motoneurons, Neuron (2023). DOI: 10.1016/j.neuron.2023.07.019